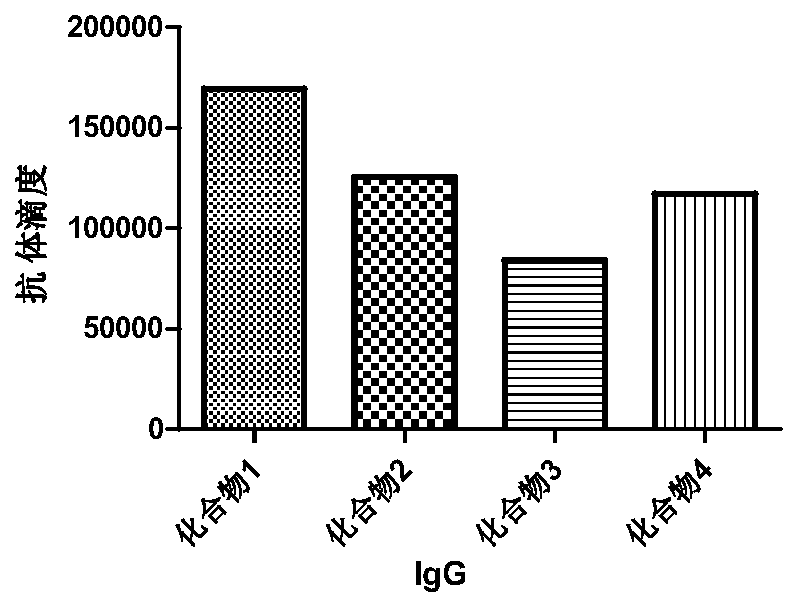
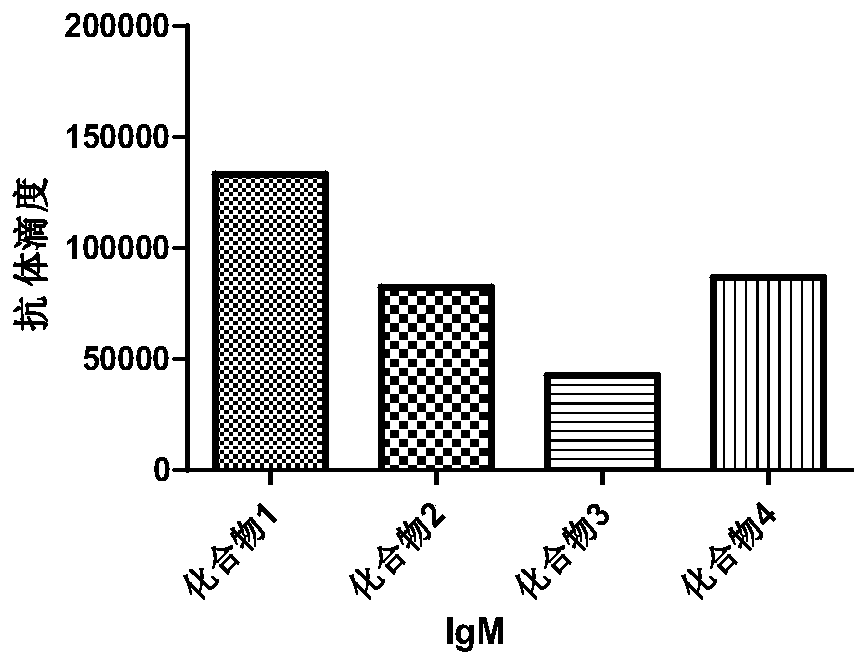
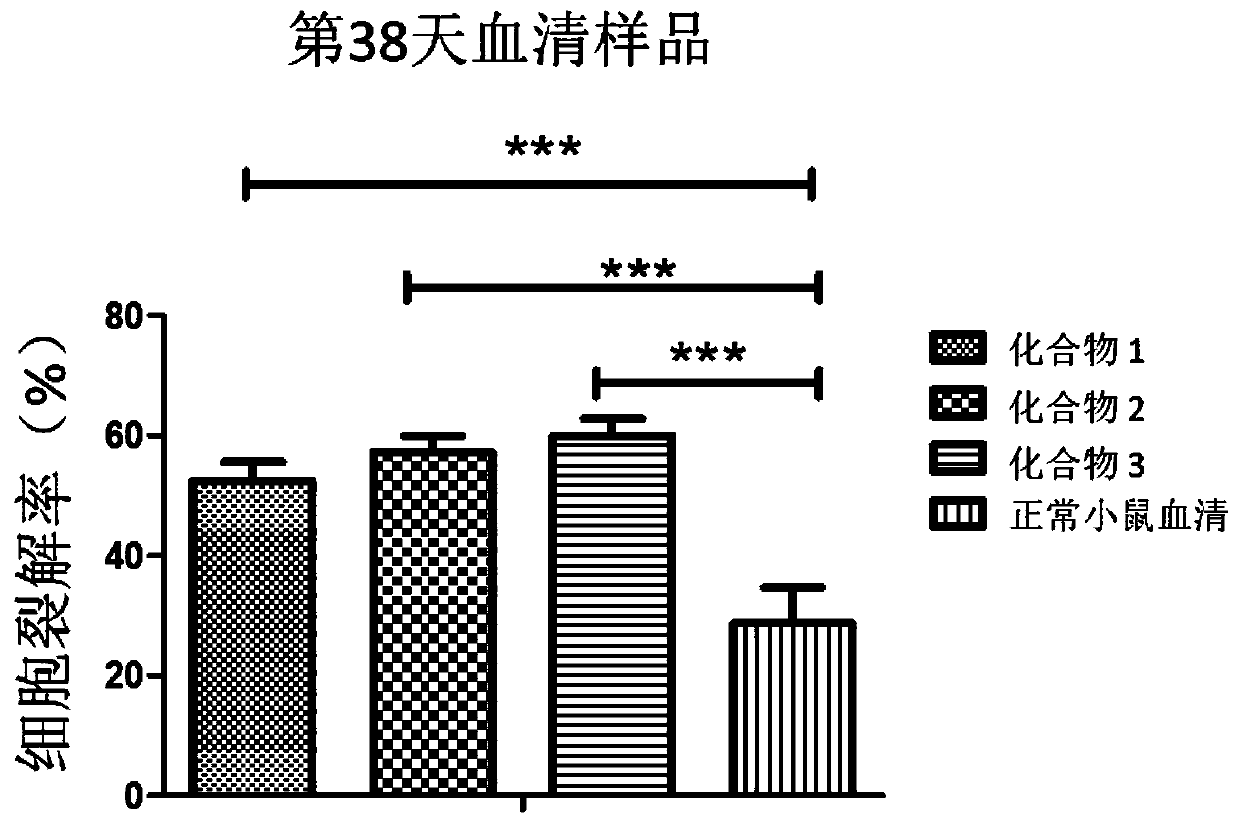
Monophosphate A conjugated-Tn anti-tumor vaccine and application thereof
A monophosphoric acid ester, anti-tumor technology, applied in the field of medicine, can solve the problems of inability to induce T cell response, poor immunogen, and inability to produce high-affinity IgG antibodies, and achieve good anti-tumor application prospects and clear structure effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of compound 1
[0053] [3-R-(dodecylcarboxylate)dodecylamino]ethyl 4-oxo-phosphoryl-6-oxo-6-{[(ethyl 2-deoxy-acetyl-α-D- Galactosyl)-1-hydrogen-1,2,3-triazolyl-4]methylene}-2-deoxy-2-[(R)-3-(tetracarboxylate) Synthesis of myristamido]-3-oxo-[(R)-3-(myristate)tetradecamido]-β-D-glucoside (compound 1)
[0054] Step 1: Preparation of 2-deoxy-2-(2-carboxybenzoyl)-D-glucose (compound 25)
[0055]
[0056] D-glucosamine hydrochloride (80.00g, 0.37mol) was added to a mixed solution of water (350.0mL) and methanol (150.0mL), the mixture was lowered to 0°C, and sodium hydroxide (15.60g , 0.39mol), after stirring in an ice bath for 1 hour, phthalic anhydride (63.20g, 0.43mol) was added, and the mixture was slowly returned to room temperature, and continued to stir overnight. (TLC) to detect the reaction process, after the detection of the complete disappearance of the raw materials, stop stirring and cool in an ice bath for 1 hour, the mixture ...
Embodiment 2
[0108] Embodiment 2: the preparation of compound 2
[0109] [3-R-(dodecylcarboxylate)tetradecylamino]ethyl 4-oxo-phosphoryl-6-oxo-6-{[(ethyl 2-deoxy-acetyl-α-D- Galactosyl)-1-hydrogen-1,2,3-triazolyl-4]methylene}-2-deoxy-2-[(R)-3-(tetracarboxylate) Synthesis of myristamido]-3-oxo-[(R)-3-(myristate)tetradecamido]-β-D-glucoside (compound 2)
[0110] Step 1: [3-R-(Dodecanoate)tetradecylamino]ethyl 4-oxo-(di-oxo-benzylphosphoryl)-6-oxo-propargyl-2-deoxy -2-[(R)-3-(tetradecyl carboxylate) tetradecyl amido]-3-oxo-[(R)-3-(tetradecyl carboxylate) tetradecyl amido]-β - Preparation of D-glucoside (compound 18)
[0111]
[0112] According to the synthesis method of step 16 in Example 1, compound 16 (0.10g, 0.07mmol) was mixed with Lipid A-2 (0.05g, 0.10mmol), N,N-diisopropylethylamine (15.3uL, 0.09mmol ) after the completion of the reaction, the crude product was obtained, and after separation and purification by column chromatography (ethyl acetate:petroleum ether=1:12, 1:8, 1:4,...
Embodiment 3
[0119] Embodiment 3: the preparation of compound 3
[0120] [3-R-(tetracarboxylate)tetradecylamino]ethyl 4-oxo-phosphoryl-6-oxo-6-{[(ethyl 2-deoxy-acetyl-α-D- Galactosyl)-1-hydrogen-1,2,3-triazolyl-4]methylene}-2-deoxy-2-[(R)-3-(tetracarboxylate) Synthesis of myristamido]-3-oxo-[(R)-3-(tetracarboxylate)tetradecamido]-β-D-glucoside (Compound 3)
[0121] Step 1: [3-R-(tetracarboxylate)tetradecylamino]ethyl 4-oxo-(di-oxo-benzylphosphoryl)-6-oxo-propargyl-2-deoxy -2-[(R)-3-(tetradecyl carboxylate) tetradecyl amido]-3-oxo-[(R)-3-(tetradecyl carboxylate) tetradecyl amido]-β - Preparation of D-glucoside (compound 19)
[0122]
[0123] According to the synthesis method of step 16 in Example 1, compound 16 (0.10mg, 0.07mmol), Lipid A-1 (0.05mg, 0.09mmol) and N,N-diisopropylethylamine (15.0uL, 0.09mmol) after the reaction was completed, the crude product was obtained, which was separated and purified by column chromatography (ethyl acetate:petroleum ether=1:12, 1:8, 1:4, 1:3, 1:2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com