Catalyst for C10+ heavy arene hydrodealkylation and preparation method thereof
A technology for hydrodealkylation and heavy aromatics, applied in physical/chemical process catalysts, molecular sieve catalysts, chemical instruments and methods, etc., can solve problems such as poor processing capacity of heavy aromatics, reduce production costs, and promote transfer. , the effect of improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
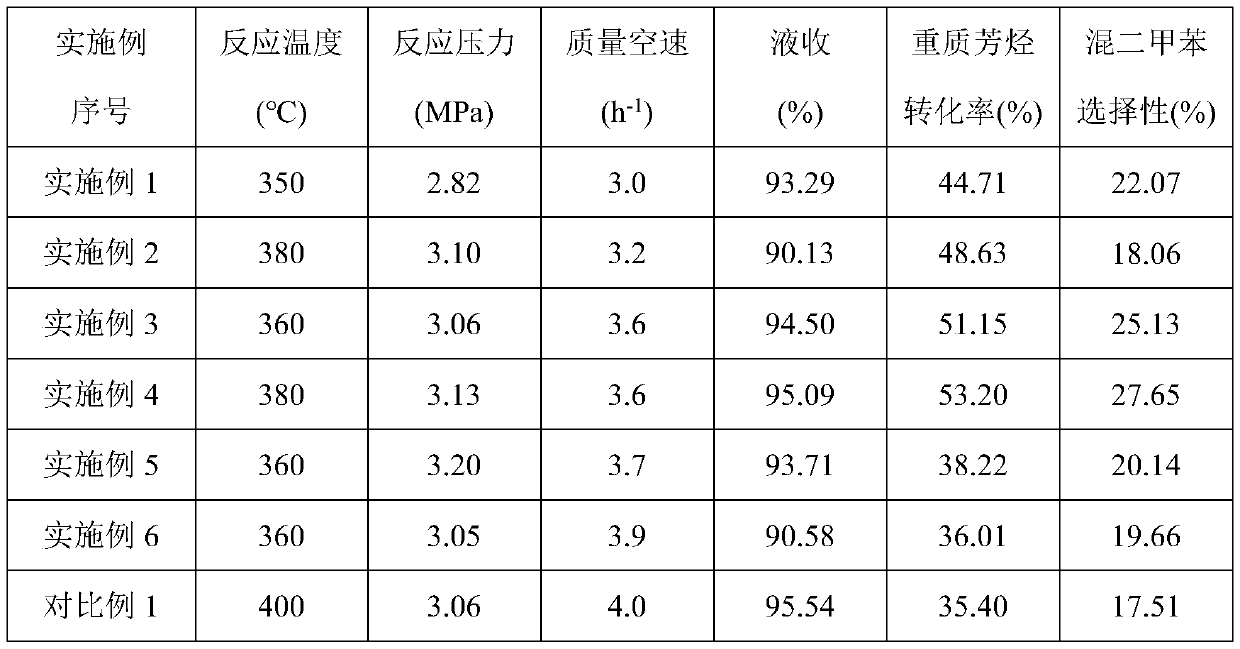
Embodiment 1
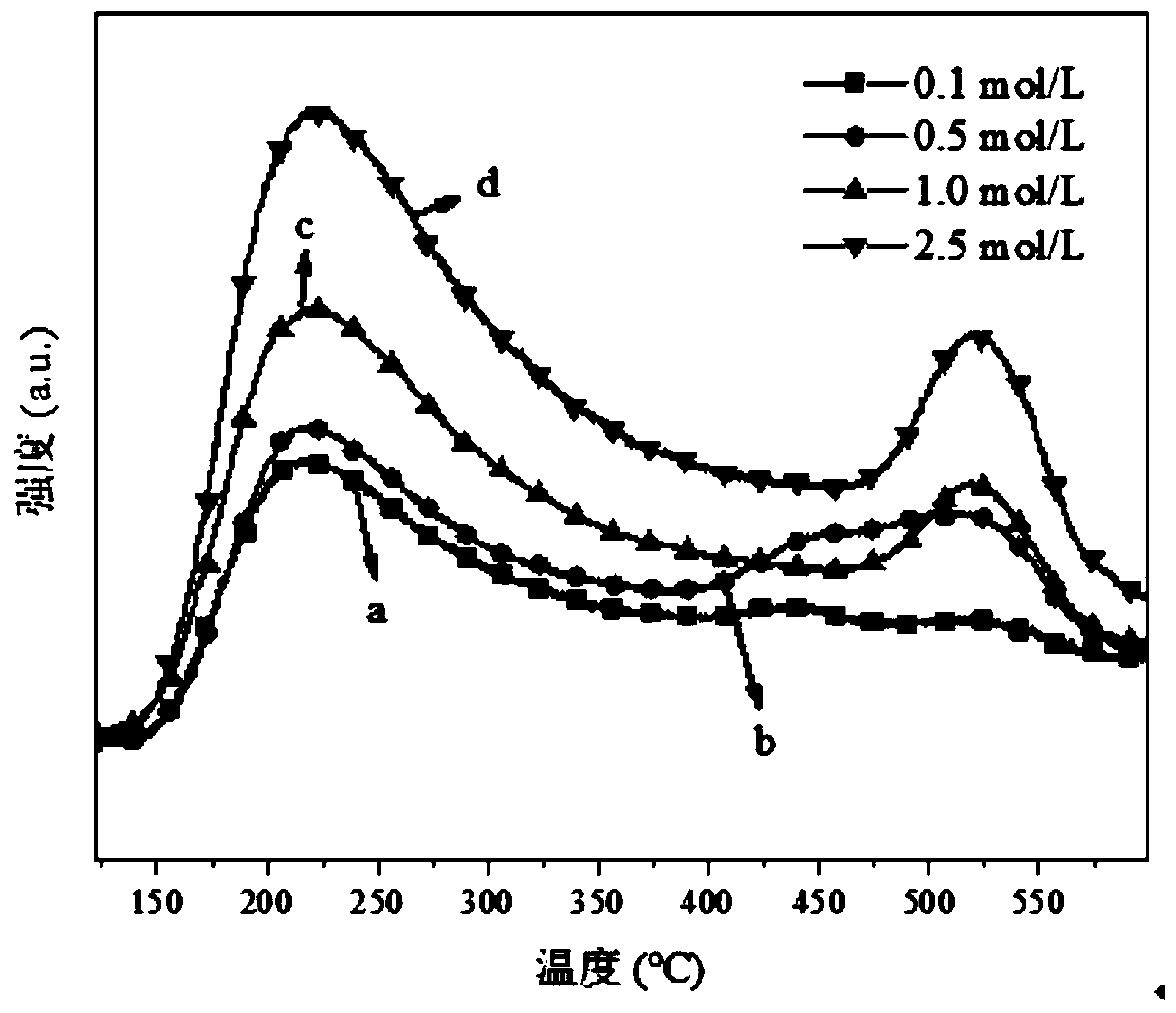
[0029] (1) Put 1.5g of β molecular sieve in 30g of 0.1mol / L hydrochloric acid solution, heat up to 60°C, stir and reflux for 2h, wash with deionized water and centrifuge until the pH is 6.6, dry at 100°C for 6h, and 400°C under air atmosphere ℃ calcination for 3 hours to obtain a modified β molecular sieve carrier with micropore-mesoporous composite structure (the specific surface area and pore volume are respectively 291.2m 2 / g and 0.30cm 3 / g).
[0030] (2) 0.12g of Ni(NO 3 )2 ·6H 2 O and 0.05g of Co(NO 3 ) 2 ·6H 2 O and 0.01g of La(NO 3 ) 3 ·6H 2 O was dissolved in 20 mL of deionized water, and a highly dispersed solution was obtained after stirring for 2 h.
[0031] (3) The molecular sieve obtained in step (1) was immersed in the precursor mixed solution obtained in step (2) for 12 hours, dried at 100° C. for 6 hours, and calcined at 400° C. for 3 hours in an air atmosphere to obtain the finished catalyst 1 . (NiO content is 2% in the catalyst, Co 3 o 4 Conten...
Embodiment 2
[0033] (1) Put 1.5g of β molecular sieve in 40g of 0.5mol / L acetic acid solution, heat up to 70°C, stir and reflux for 3h, wash with deionized water and centrifuge until the pH is 6.9, dry at 110°C for 7h, and 450°C under air atmosphere ℃ calcination for 3.5h to obtain a modified β molecular sieve carrier with a microporous-mesoporous composite structure (the specific surface area and pore volume are respectively 295.8m 2 / g and 0.32cm 3 / g).
[0034] (2) 0.24g of Ni(NO 3 ) 2 ·6H 2 O and 0.04g of (NH 4 ) 6 Mo 7 o 24 4H 2 O and 0.03g of Ce(NO 3 ) 3 ·6H 2 O was dissolved in 20 mL of deionized water, and a highly dispersed solution was obtained after stirring for 2.5 h.
[0035] (3) The molecular sieve obtained in step (1) was immersed in the precursor mixed solution obtained in step (2) for 14 hours, dried at 110°C for 8 hours, and calcined at 450°C for 3.5 hours in an air atmosphere to obtain the finished catalyst 2. (NiO content is 4% in the catalyst, MoO 3 Conte...
Embodiment 3
[0037] (1) Place 1.5g of β molecular sieve in 50g of 1.0mol / L mixed solution of nitric acid and hydrochloric acid, heat up to 85°C, stir and reflux for 3.5h, wash and centrifuge with deionized water until the pH is 7.0, and dry at 120°C for 12h. Calcined at 500°C for 4 hours in an air atmosphere to obtain a modified β molecular sieve carrier with a microporous-mesoporous composite structure (the specific surface area and pore volume are 298.0m 2 / g and 0.35cm 3 / g).
[0038] (2) 0.48g of Ni(NO 3 ) 2 ·6H 2 O and 0.08g of (NH 4 ) 6 Mo 7 o 24 4H 2 O and 0.03g of Ce(NO 3 ) 3 ·6H 2 O was dissolved in 20 mL of deionized water, and a highly dispersed solution was obtained after stirring for 3 h.
[0039] (3) The molecular sieve obtained in step (1) was immersed in the precursor mixed solution obtained in step (2) for 16 hours, dried at 120° C. for 10 hours, and calcined at 500° C. for 4 hours in an air atmosphere to obtain the finished catalyst 3 . (NiO content in the ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com