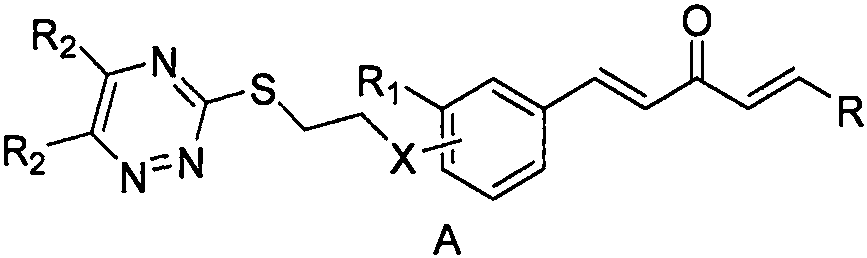
Pentadienone compound containing triazine, and preparation method and application of pentadienone compound
A kind of ketone compound, pentadiene technology, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
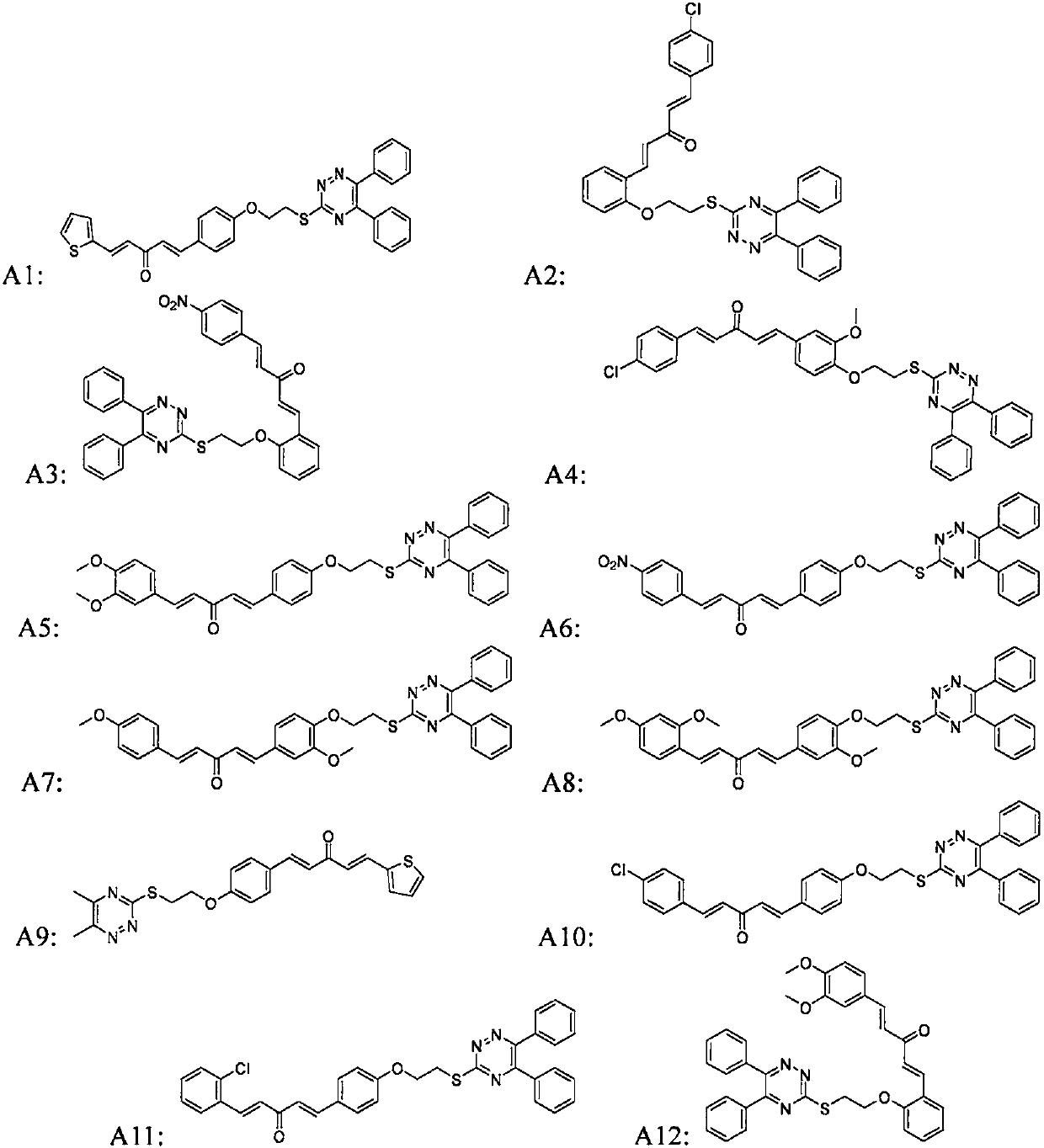
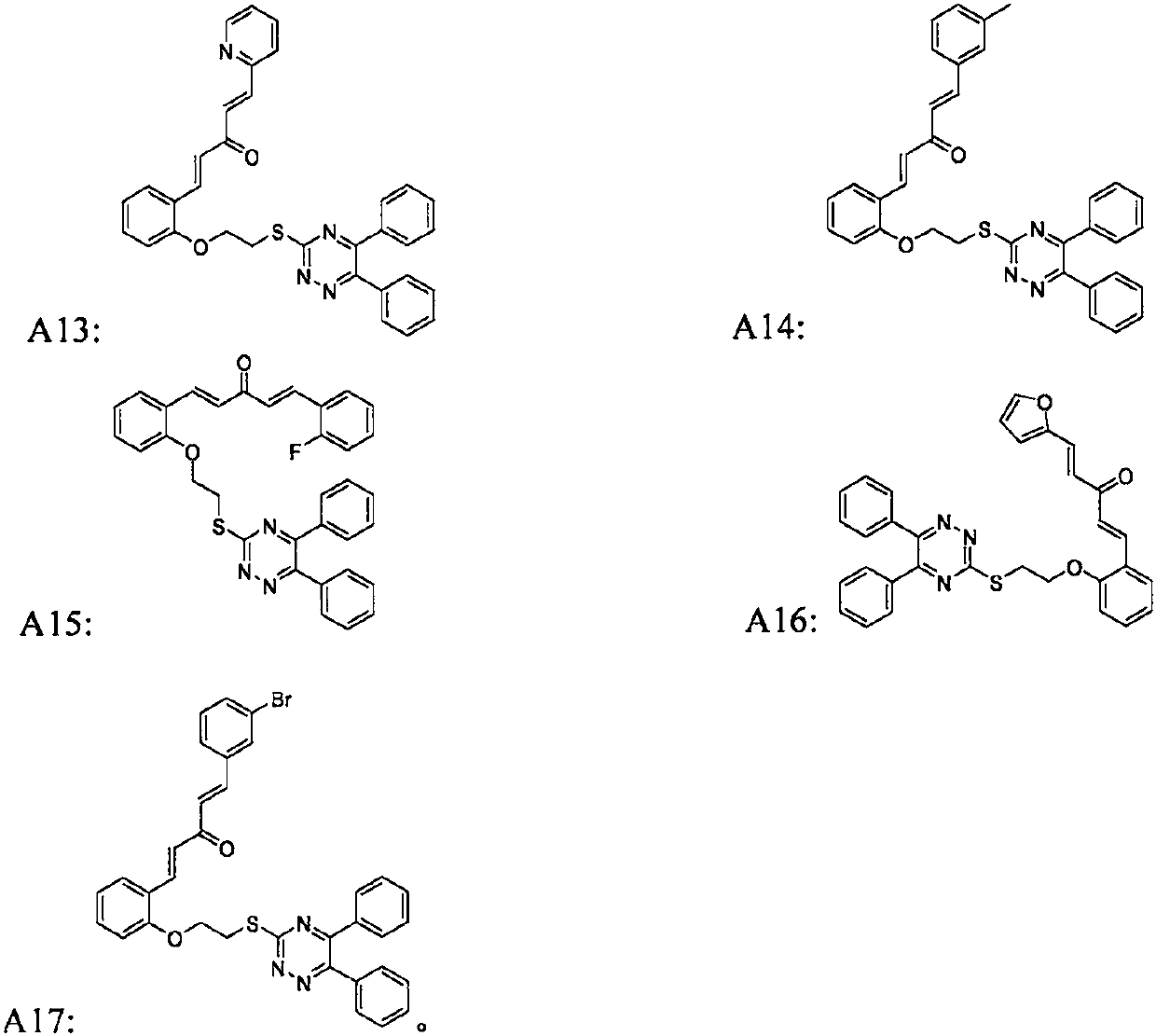
[0077] (1E,4E)-1-(4-(2-((5,6-diphenyl-1,2,4-triazine-3-mercaptoethoxy)phenyl)-5-(2-thiophene )-5-substituted-1,4-pentadien-3-one (target compound A1):
[0078]
[0079] The preparation method comprises the following steps:
[0080] (1) Preparation of 5,6-diphenyl-1,2,4-triazine-3-mercaptool (intermediate 1):
[0081] Benzil (1.5g) and 35mL of glacial acetic acid were added to a 100mL three-necked flask, and then thiosemicarbazide (0.65g) was dissolved in 25mL of hot water, and the mixed system was added to the three-necked flask. Stir at ℃ for 4 h, and after the reaction is completed, filter while heating to obtain a yellow solid with a yield of 78%.
[0082] (2) Preparation of 4-(hydroxyphenyl)-3-buten-2-one (intermediate 2):
[0083] 4-Hydroxybenzaldehyde (6.1g) is added in the acetone of 60mL, after stirring for about 15min, after ice-bathing the reaction system for about 30min, add about 100mL of 5% NaOH solution in the system, after the dropwise addition is complete...
Embodiment 2
[0091] (1E, 4E)-1-(2-(2-((5,6-diphenyl-1,2,4-triazine-3-mercaptoethoxy)phenyl)-5-(4-chloro Phenyl)-1,4-pentadien-3-one (target compound A2):
[0092]
[0093] The target compound A2 was prepared by selecting corresponding raw materials in the steps similar to Example 1, and the yield was 56%.
Embodiment 3
[0095] (1E, 4E)-1-(2-(2-((5,6-diphenyl-1,2,4-triazine-3-mercaptoethoxy)phenyl)-5-(4-nitro phenyl)-1,4-pentadien-3-one (target compound A3):
[0096]
[0097] The target compound A3 was prepared by selecting corresponding raw materials according to the steps similar to Example 1, and the yield was 35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com