Antitumor protein and application thereof
A protein and anti-tumor drug technology, applied in the field of anti-tumor drugs, can solve the problems of poor effect of solid tumors, high risk of surgical treatment, adverse reactions of radiotherapy and chemotherapy, etc., achieve good disease control, improve quality of life, and mild adverse reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
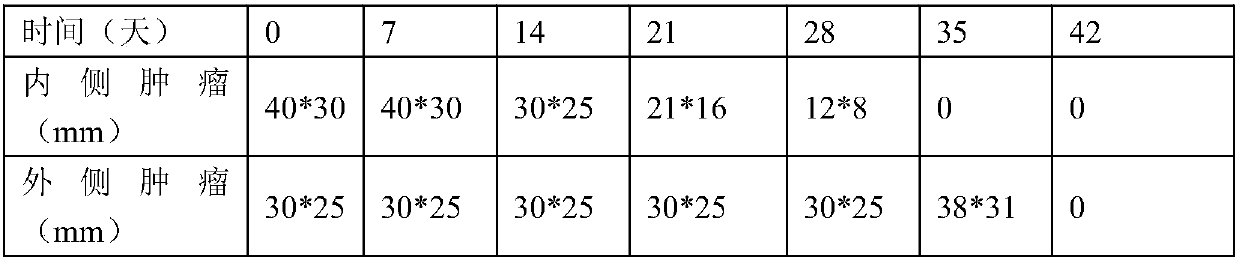
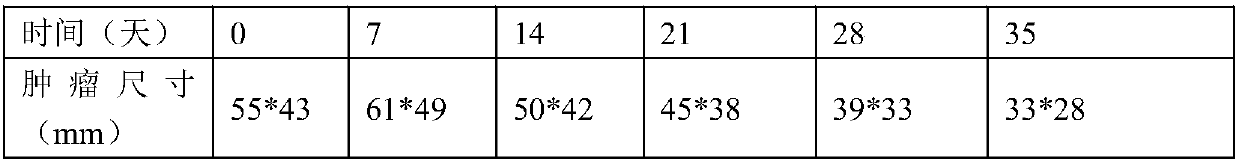
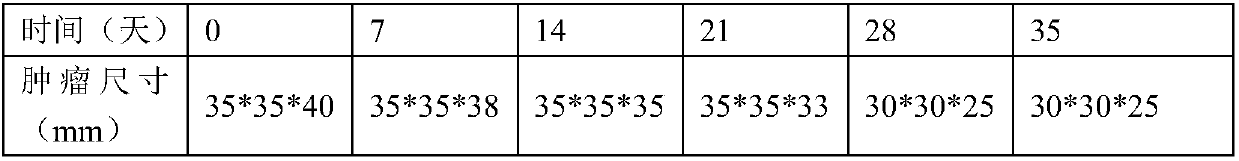
Examples
Embodiment 1IL12
[0027] Example 1 Construction of IL12 expressing cells
[0028] The coding region of the canine IL12 gene was synthesized, including IL12α (Genbank ID: NM_001003293) and IL12β (Genbank ID: NM_001003292) two subunits, connected with T2A sequence in the middle, and the two ends of the synthesized gene had BamHI and XhoI restriction sites respectively Spot, then digest with BamHI and XhoI, the system is as follows: 5 μg of IL12 plasmid, 4 μL of digestion buffer, 1 μL of BamHI, 1 μL of XhoI, add water to a total volume of 40 μL, and let stand at 37°C for 12 hours. Take out the EP tube, add 4.4 μL of 10× sample buffer, and perform electrophoresis on 1% agarose gel. After electrophoresis, the IL12 gene fragment is recovered for use.
[0029] Digest the expression vector pLentis-CMV-MCS-IRES-PURO, and the system is as follows: plasmid 2 μg, enzyme digestion buffer 3 μL, BamHI 1 μL, XhoI 1 μL, add water to a total volume of 30 μL, and stand at 37°C for 12 hours. Take out the EP tube,...
Embodiment 2
[0033] Example 2 GMCSF expressing cell construction
[0034]Synthesize the coding region of the canine GMCSF (Genbank number: NM_001003245) gene, with BamHI and XhoI restriction sites at both ends of the synthesized gene, and then digest with BamHI and XhoI. The system is as follows: 5 μg of GMCSF plasmid, digestion buffer 4 μL, BamHI 1 μL, XhoI 1 μL, add water to a total volume of 40 μL, and let stand at 37°C for 12 hours. Take out the EP tube, add 4.4 μL of 10× sample buffer, and perform electrophoresis on 1% agarose gel. After electrophoresis, the GMCSF gene fragment is recovered for use.
[0035] Digest the expression vector pLentis-CMV-MCS-IRES-PURO, and the system is as follows: plasmid 2 μg, enzyme digestion buffer 3 μL, BamHI 1 μL, XhoI 1 μL, add water to a total volume of 30 μL, and stand at 37°C for 12 hours. Take out the EP tube, add 3.3 μL of 10 × sample buffer, and run electrophoresis on 1% agarose gel. After electrophoresis, the carrier fragments are recovered a...
Embodiment 3I
[0039] Example 3 Construction of IL2 expressing cells
[0040] Synthesize the coding region of the canine IL2 (Genbank number: NM_001003305) gene, with BamHI and XhoI restriction sites at both ends of the synthesized gene, and then digest with BamHI and XhoI. The system is as follows: IL2 plasmid 5 μg, digestion buffer 4 μL, BamHI 1 μL, XhoI 1 μL, add water to a total volume of 40 μL, and let stand at 37°C for 12 hours. Take out the EP tube, add 4.4 μL of 10× sample buffer, and run electrophoresis on 1% agarose gel. After electrophoresis, the IL2 gene fragment is recovered for use.
[0041] Digest the expression vector pLentis-CMV-MCS-IRES-PURO, and the system is as follows: plasmid 2 μg, enzyme digestion buffer 3 μL, BamHI 1 μL, XhoI 1 μL, add water to a total volume of 30 μL, and stand at 37°C for 12 hours. Take out the EP tube, add 3.3 μL of 10 × sample buffer, and run electrophoresis on 1% agarose gel. After electrophoresis, the carrier fragments are recovered and set asi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com