Antimicrobial preparations
A technology of bactericidal effect and preparation, applied in the medical field, can solve the problems of inability to directly interact with viruses and microorganisms, and low efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1. Synthetic Diffus antiviral peptide:
[0017] -HN 2 -His-gly-val-ser-gly-his-gly-gln-his-gly-val-his-gly-coOh (1)
[0018] As a temporary protecting group, a tert-butyloxycarbonyl group is used. A histidine and serine benzyl dinitrophenyl group were used as a permanent protected group. Synthesis is carried out on a benzoyl methyl polymer having a capacity of 0.8 mmol / g. As shown in Table 1, the peptide chain is gradually increased from the C end.
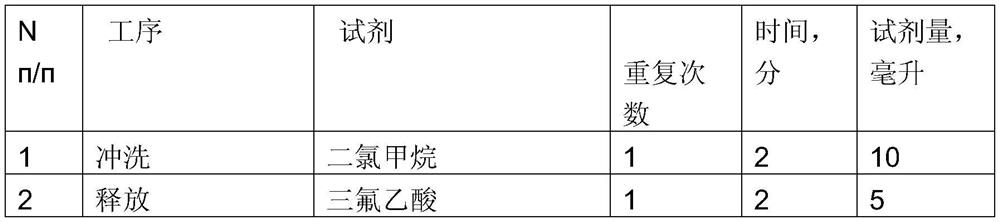
[0019] Table 1 Anti-adhesion procedure for amino acid residues
[0020]
[0021]
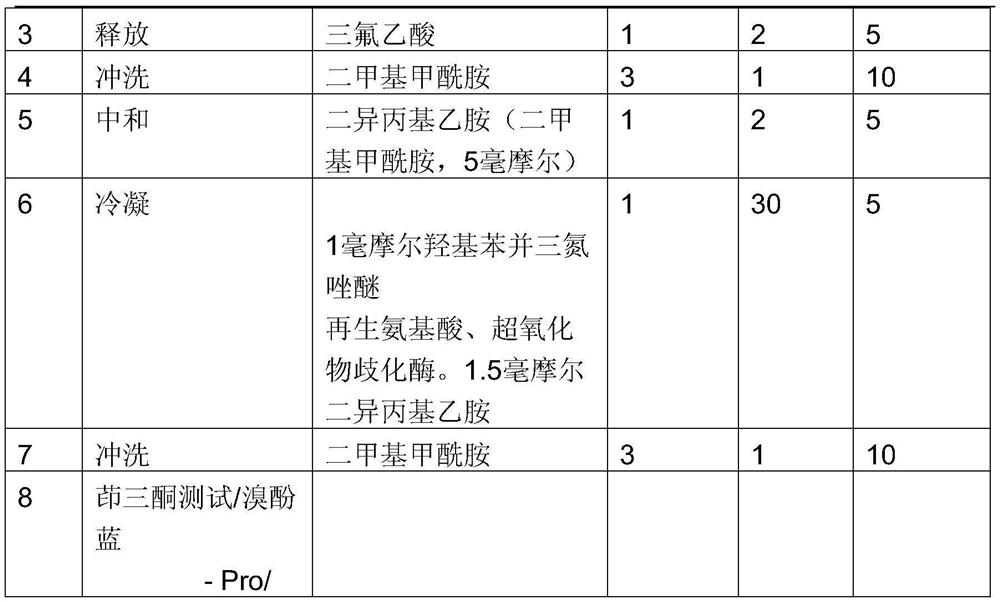
[0022] If the ninluol is detected, the cycle step is repeated from the third step.
[0023] After the synthesis of peptide-based polymers, 50% trifluoroacetic acid was treated with dichloromethane, and then washed with 5 ml of dichloromethane and removed from the container. It was rinsed with 5 ml of isopropyl alcohol and washed 5 ml of anhydrous ethyl ether three times. The obtained peptide-based polymer was dried in a vacuum dryer...
Embodiment 2
[0028] Synthetic synthetic peptide:
[0029] HN 2 - (CH 2 ) 10 -CO-ILE-LEU-PRO-D-PHE-LYS-D-Phe-PRO-D-PHE-D-Phe-PRO-D-Phe-Arg-Arg-NH 2 (2)
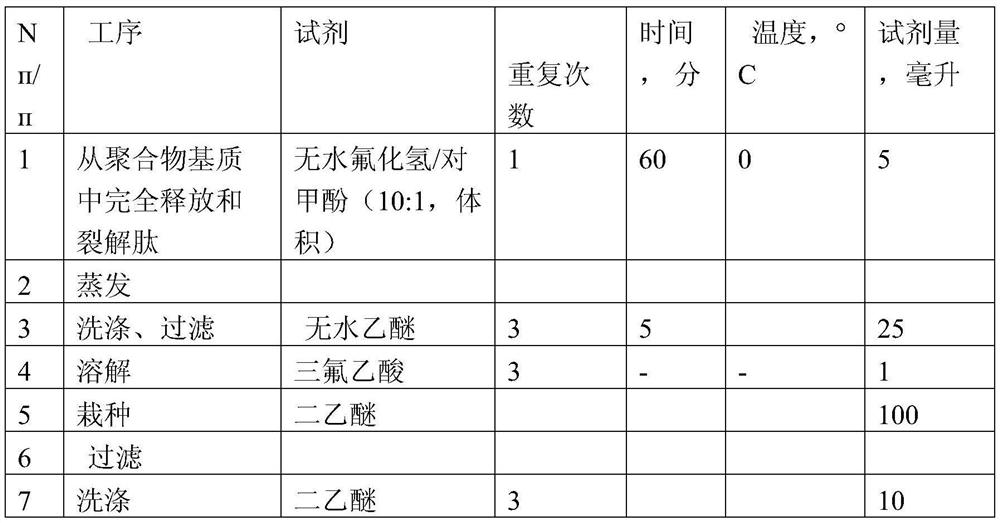
[0030] The synthesis, release, and cleaning methods of peptide (2) are the same as those of peptide 1. Protect nitro arginine N ω Function uses ε-amino groups of lysine, chloroformate group. In order to synthesize peptide in the reaction kettle, 10 ml of dimethylformamide was added to 0.2 g N-tert-butyl hydroxyl group-N ω Nitro-amanine methyl amphorne. The arginine derivative is 1 mmol / gram polymer.
[0031] After the polypeptide chain is completed, the protective peptide polymer is released while the polymer and the peptide are obtained using a liquid fluoride fluoride. The gradient of acetonitrile was purified at 0.1% trifluoroacetic acid during purification. Basic substance - peptide content HN 2 - (CH 2 ) 10 -CO-ILE-LEU-PRO-D-PHE-LYS-D-Phe-PRO-D-PHE-D-Phe-PRO-D-Phe-Arg-Arg-NH 2 - According to the optical density data, at least 99%. The a...
Embodiment 3
[0032]Example 3. Preparation of gels for skin and mucosal diseases.
[0033] Preparation of gels use chemical reactors and agitators. Put (polycarboxy) (1.5-2.0%) distilled water, NaOH solution (1.5-2.0%)), which was continued for 30 minutes. Then, the pH 6 was adjusted to pH 6, stirred, and the aqueous solution containing a preservative was injected, and methyl hydroxybenzoate (0.01-0.2% by mass) and hydroxybenzate (0002-0.01) were used as preservative. quality%). After the mixture was produced, 1.5-2.5% by mass of glycerol was added. Then it is stirred for 10 minutes and the pH is measured. The gel was then placed once a day, then retransmit, and evenly bracket in the container (tube). Thus, a gel having a homogeneous transparent and abutment of foreign particles is obtained. The gel component (mass%) is as follows: peptide -0, 02-0, 2; excipients - 99, 98-99, 8. Characteristics of peptid gel formulations are shown in Table 3.
[0034] Table 3 Components with peptide gel product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com