Acetaminophen and tramadol co-soluble compound analgesic oral liquid
An analgesic oral liquid and acetaminophen technology, which can be applied in the directions of non-central analgesics, pharmaceutical formulations, dispersion liquid delivery, etc., can solve the problems of patient burden, difficulty in using acetaminophen and tramadol compound oral liquid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] This example is about judging whether the co-dissolution of acetaminophen and tramadol has been completed and presented as a "clear and transparent solution". Whether it appears as a "clear and transparent solution" is judged by visual identification. After each formulation is prepared in the transparent centrifuge tube and left to stand for 24 hours, the bottom of the centrifuge tube is visually observed. If there is no sediment or floating matter at the bottom of the centrifuge tube, it is judged as "clear and transparent solution".
[0020] If the formation of powdery precipitates or cotton flocs is observed at the bottom of the centrifuge tube, it is judged as "incompletely dissolved".
[0021] If the formula is clear and transparent when the transparent centrifuge tube is prepared, but after standing for 24 hours, a precipitate with crystal form is observed at the bottom of the centrifuge tube, then it is judged as "recrystallization". In addition, in order to fu...
Embodiment 2
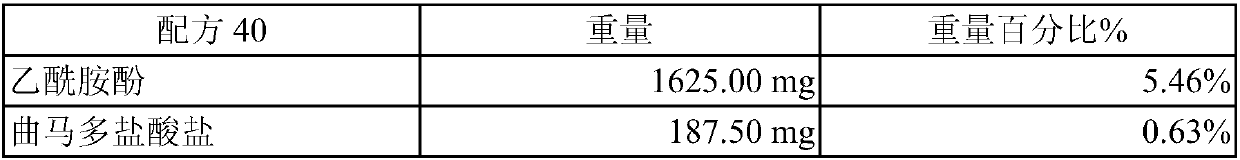
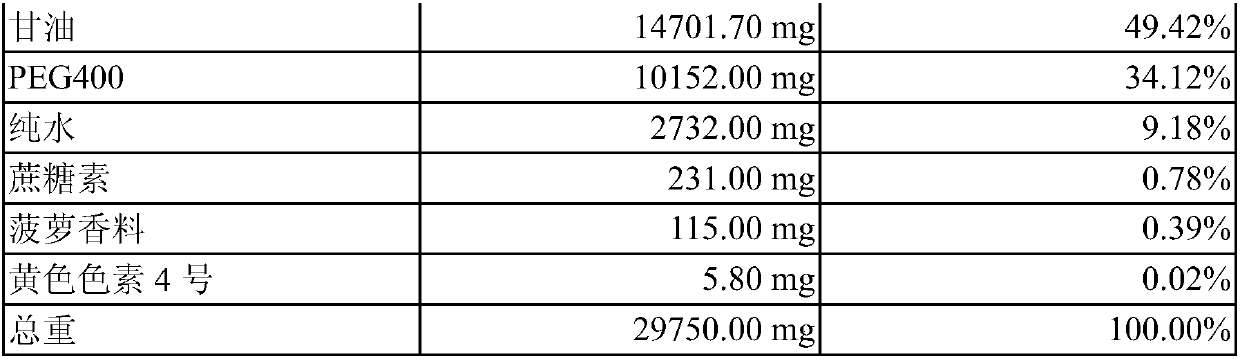
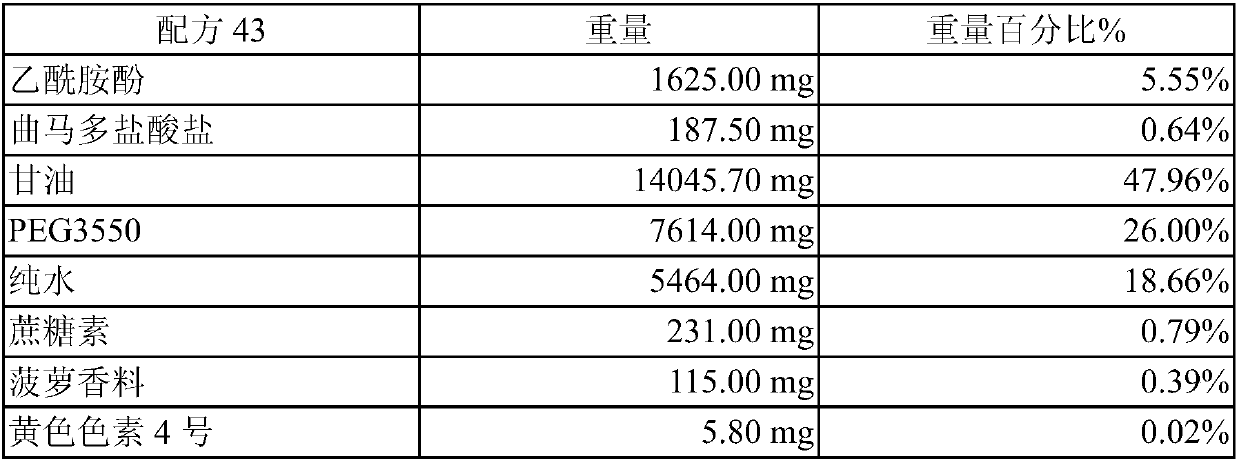
[0025] This example is about the manufacture of the co-solvent of acetaminophen and tramadol and the determination of whether it is a "clear and transparent solution". In this embodiment, glycerin and pure water are used as the main components of the solvent system, and propylene glycol and different polyethylene glycols (Polyethylene glycol hereinafter referred to as PEG) are used as co-solvents. The PEGs used in this embodiment are PEG400, PEG1000 and PEG3550 (polyethylene glycol 400, polyethylene glycol 1000 and polyethylene glycol 3550). When preparing, first mix pure water with glycerin and / or propylene glycol to form a uniform mixed solution, then slowly add acetaminophen, tramadol and other ingredients and use ultrasonic vibration to dissolve them. This embodiment judges whether it is a "clear and transparent solution" by the method shown in the foregoing embodiment 2, and exemplifies the following formulas 37, 39 to 43:
[0026] Recipe 37 weight % by weig...
Embodiment 3
[0045]This example is about the manufacture of acetaminophen and tramadol co-solvent and the determination of whether it is a "clear and transparent solution". In this embodiment, glycerin and pure water are used as the main components of the solvent system, and PEG400 is used as a co-solvent. When preparing, first mix pure water and glycerin to form a homogeneous mixed solution, then slowly add acetaminophen, tramadol and other ingredients and use ultrasonic vibration to dissolve them. This embodiment judges whether it is a "clear and transparent solution" by the method shown in the foregoing embodiment 1, and exemplifies the following formula 46:
[0046] Recipe 46 weight % by weight Acetaminophen 1625.00mg 5.45% tramadol hydrochloride 187.50mg 0.63% glycerin 14639.70mg 49.11% PEG400 10152.00mg 34.06% pure water 2794.00 mg 9.37% Sucralose 231.00mg 0.77% pineapple spice 115.00mg 0.39% Yellow Pigment No...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com