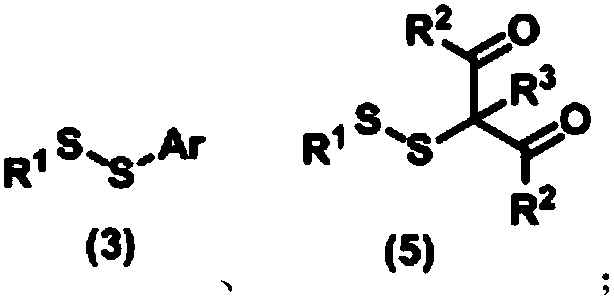
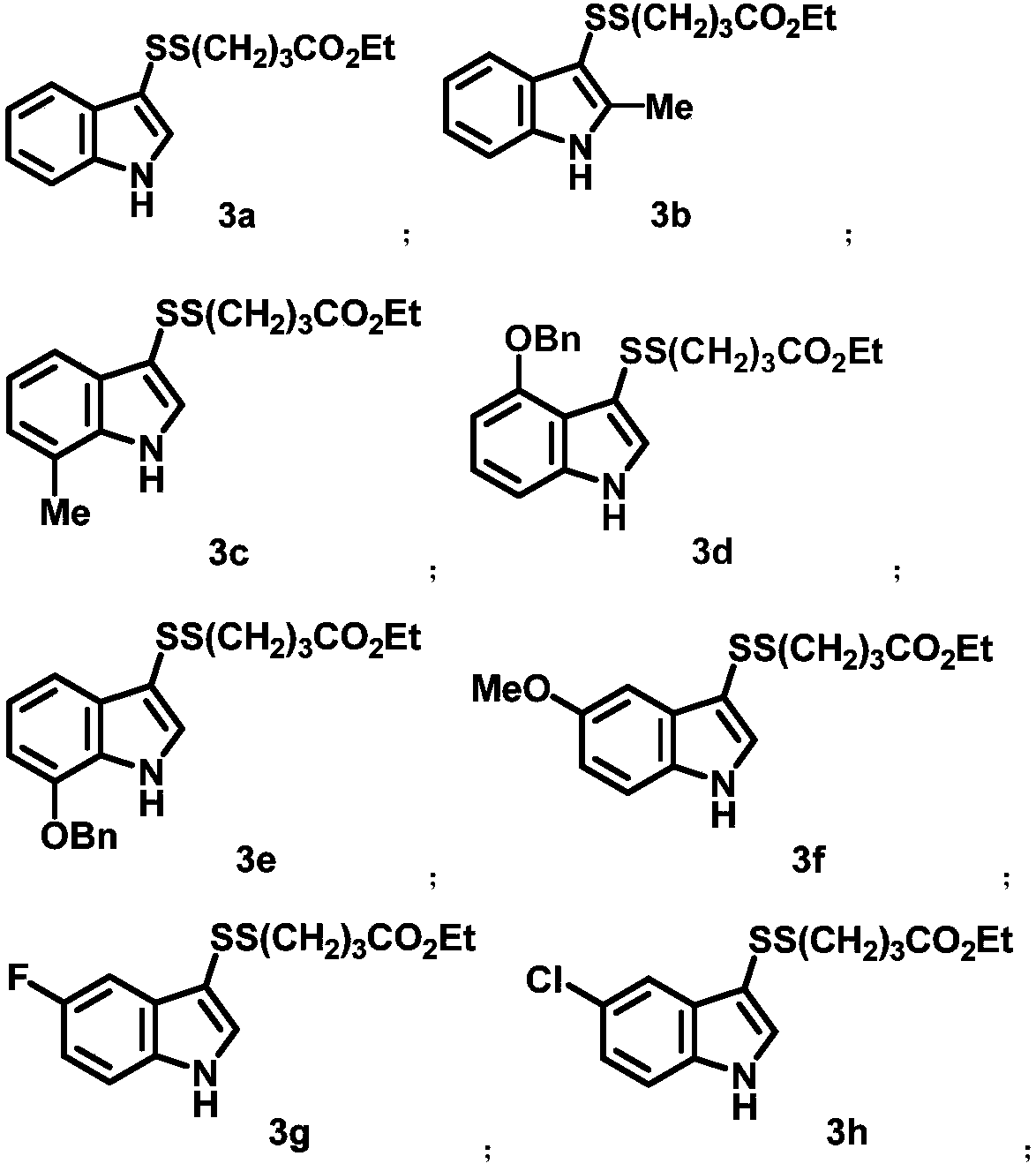
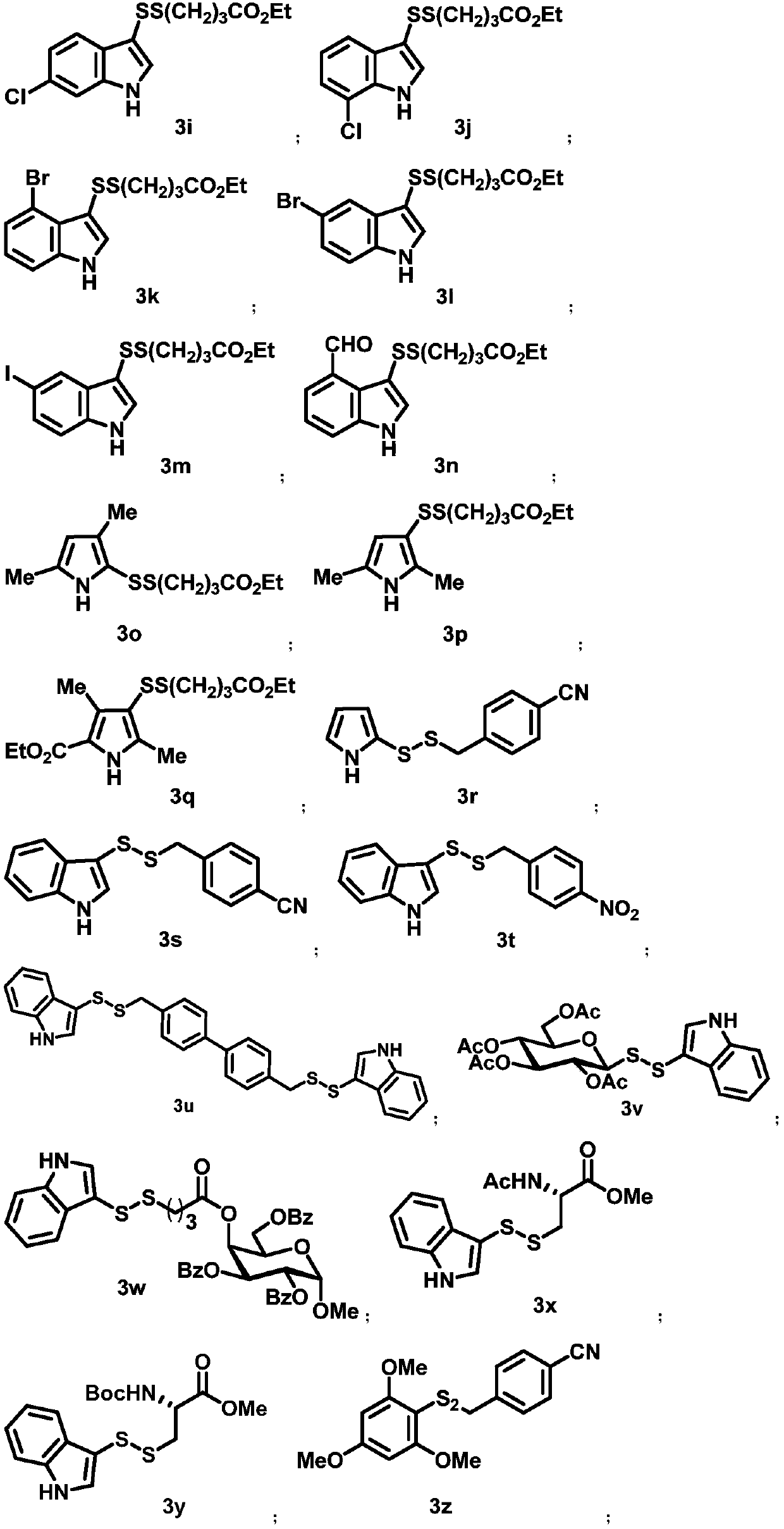
Asymmetric disulfide compound and synthesis method and application thereof
A synthetic method and asymmetric technology, applied in the field of asymmetric disulfide compounds and their synthesis, can solve the problems of environmental and human injury, easy to be oxidized, poisoning of metal catalysts, etc., and achieve the effect of solving electrophilic persulfide reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Synthesis of compound 3a:
[0084]
[0085] Under air atmosphere, 1a (42.1mg, 0.2mmol), indole (35.1mg, 0.3mmol), methanesulfonic acid (2.0mg, 0.02mmol), tert-amyl alcohol (0.5mL) were successively added to the reaction tube, and the reaction system Stirring at 0°C for 5 hours, after the reaction was completed, dichloromethane was diluted, the solvent was removed, and column chromatography gave white solid compound 3a (46.0 mg, 78%). 1 H NMR (400MHz, CDCl 3 )δ8.47(s,1H),7.83-7.81(m,1H),7.38-7.34(m,2H),7.31–7.17(m,2H),4.12(q,J=7.1Hz,2H),2.76 (t, J=7.0Hz, 2H), 2.41(t, J=7.3Hz, 2H), 2.10(p, J=7.2Hz, 2H), 1.24(t, J=7.1Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ173.12,136.28,128.59,123.14,121.03,119.51,111.56,10806,60.39,37.54,32.73,23.77,14.22.IR(film)3398,2926,1714,1497,14753,1411,373,137,1 ,1131,1095,1035,745. HRMS (EI) Calcd for C 15 h 19 NO 2 S 2 295.0701,Found 295.0705.
Embodiment 2
[0087] Synthesis of compound 3a:
[0088]
[0089] Under air atmosphere, 1a (42.1mg, 0.2mmol), indole (35.1mg, 0.3mmol), camphorsulfonic acid (4.6mg, 0.02mmol), 1,2-dichloroethane (0.5 mL), the reaction system was stirred at 0°C for 5 hours, after the reaction was completed, dichloromethane was diluted, the solvent was removed, and column chromatography gave white solid compound 3a (29.5mg, 50%).
Embodiment 3
[0091] Synthesis of compound 3a:
[0092]
[0093]Under air atmosphere, add 1a (42.1mg, 0.2mmol), indole (35.1mg, 0.3mmol), o-nitrobenzenesulfonic acid (4.1mg, 0.02mmol), 1,2-dichloroethyl Alkane (0.5mL), the reaction system was stirred at 0°C for 5 hours, after the reaction was completed, dichloromethane was diluted, the solvent was removed, and column chromatography gave white solid compound 3a (30.7mg, 52%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com