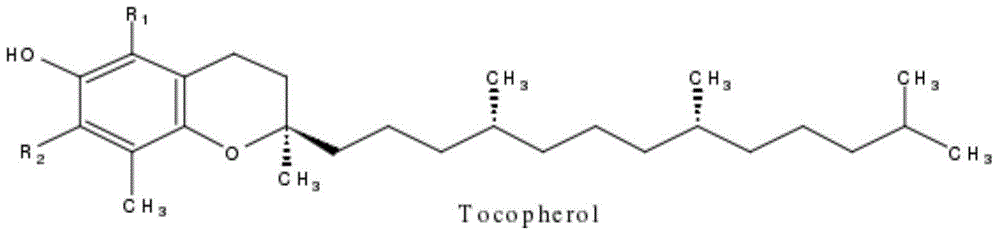
Separation method of tocopherol homologous compounds
A separation method and technology of tocopherol, which is applied in the field of lipid processing, can solve the problems of large preparation volume and strong load capacity, and achieve the effects of less sample loss, convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
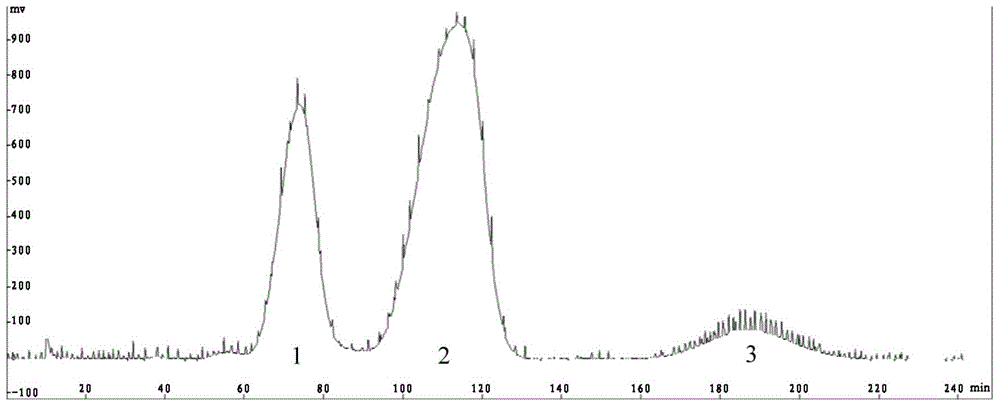
[0080] The tocopherol mixture used in this example is mixed tocopherol. As determined by HPLC method, the total content of α-tocopherol, (β+γ)-tocopherol and δ-tocopherol is 96.5wt%. Among them, α-tocopherol The content of phenol was 22.9 wt%; the content of (β+γ)-tocopherol was 35.8 wt%; the content of δ-tocopherol was 37.8 wt%.
[0081] (1) According to the ratio of n-heptane: ethanol: water = 5:5:1 (v / v / v), the above three solvents are added to the separatory funnel, shaken 2-3 times and fully mixed, then stand to separate phase, to obtain a two-phase mixed solution; use a clean reagent bottle to collect the upper phase solvent and the lower phase solvent in the two-phase mixed solution, put them into an ultrasonic oscillator for degassing treatment, stand still after degassing for 25 minutes, and wait for The solvent was returned to room temperature.
[0082] (2) Take 1.052g of tocopherol mixture and dissolve it in 20ml of the upper phase solvent obtained in step (1) as t...
Embodiment 2
[0087] The tocopherol mixture used in this example is mixed tocopherol, and the total content of α-tocopherol, (β+γ)-tocopherol and δ-tocopherol is 92.1wt% as determined by HPLC method, wherein α-tocopherol The content of phenol was 21.8 wt %; the content of (β+γ)-tocopherol was 33.4 wt %; the content of δ-tocopherol was 36.9 wt %.
[0088] (1) According to the ratio of n-hexane: methanol: water = 30:30:0.5 (v / v / v), add the above three solvents to the separatory funnel, shake for 2-3 times and mix thoroughly, then let stand for phase separation , to obtain a two-phase mixture; use a clean reagent bottle to collect the upper phase solvent and the lower phase solvent in the two-phase mixture, put them into an ultrasonic oscillator for degassing treatment, stand still after degassing for 25 minutes, and wait for the solvent Return to room temperature.
[0089] (2) Take 1.034g of tocopherol mixture and dissolve it in 20ml of the upper phase solvent obtained in step (1) as the sam...
Embodiment 3
[0094] The tocopherol mixture used in this example is mixed tocopherol, and the total content of α-tocopherol, (β+γ)-tocopherol and δ-tocopherol is 52.8wt% as determined by HPLC method, wherein α-tocopherol The content of phenol was 7.8 wt%; the content of (β+γ)-tocopherol was 30.4 wt%; the content of δ-tocopherol was 14.6 wt%.
[0095] (1) According to the ratio of n-heptane:methanol:water=25:25:1 (v / v / v), the above three solvents are added to the separatory funnel, shaken 2-3 times and fully mixed, then stand to separate phase, to obtain a two-phase mixed solution; use a clean reagent bottle to collect the upper phase solvent and the lower phase solvent in the two-phase mixed solution, put them into an ultrasonic oscillator for degassing treatment, stand still after degassing for 25 minutes, and wait for The solvent was returned to room temperature.
[0096] (2) Take 1.003g of tocopherol mixture and dissolve it in 20ml of the upper phase solvent obtained in step (1) as the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com