Double-layer artificial blood vessel and preparation method thereof
An artificial blood vessel, double-layer technology, applied in the field of functional double-layer artificial blood vessels, can solve the problems of intimal hyperplasia, easy formation of thrombus, calcification, etc., to promote the regulation of inflammatory factor expression, excellent mechanical properties, and promote rapid growth Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
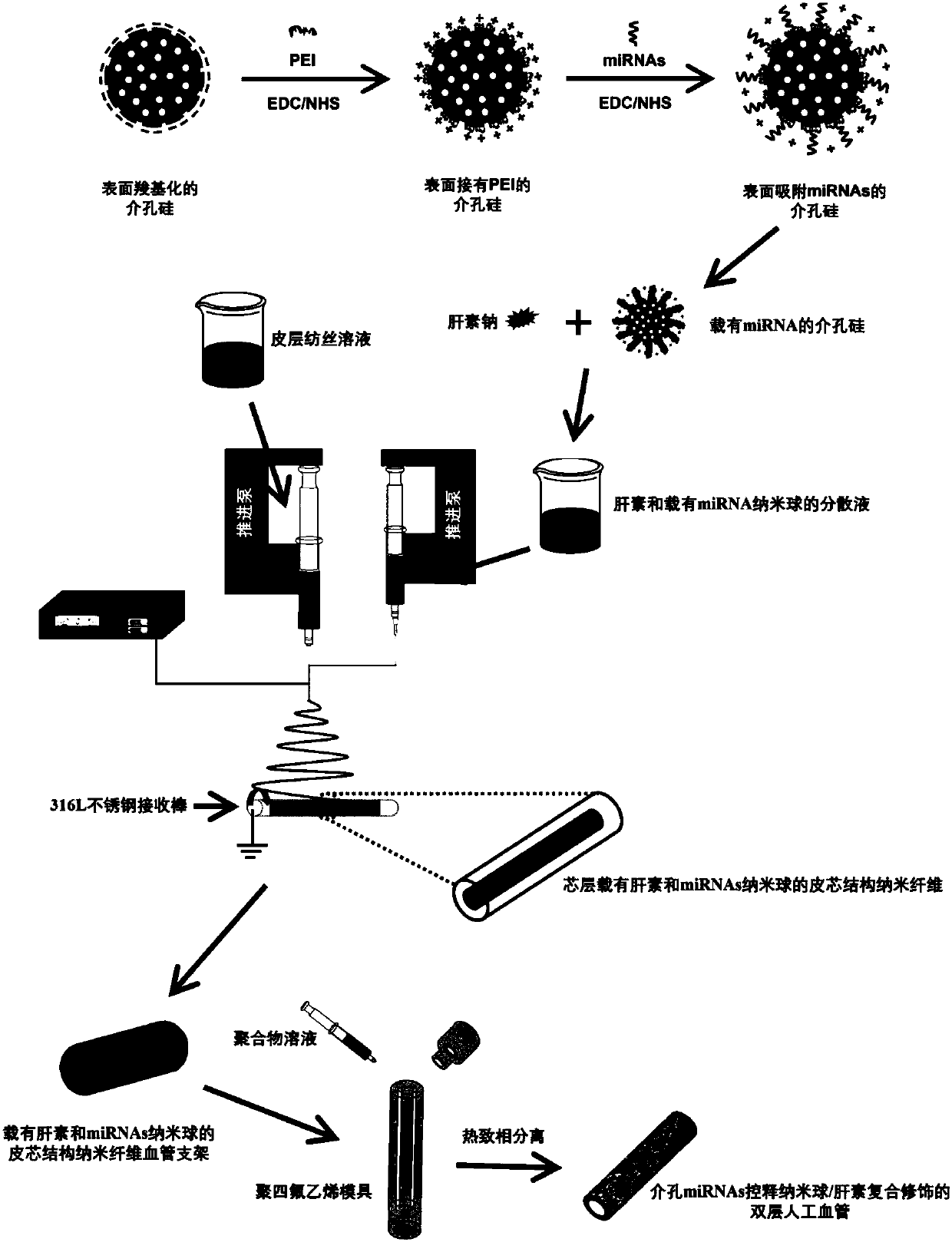
[0058] 1) Disperse 50 mg of surface carboxylated mesoporous silicon (MSNs-COOH) into 50 mL of dimethyl sulfoxide (DMSO), and disperse it evenly by ultrasonication;
[0059] 2) Weigh a total amount of 0.8g of EDC and NHS with a molar ratio of 2.5:1 and add them to the above dispersion, and stir at 32°C for 2 hours to activate the carboxyl groups on MSNs-COOH;
[0060] 3) Add 50mL polyethyleneimine (PEI) aqueous solution with a concentration of 80mg / mL dropwise to the carboxyl-activated MSNs-COOH dispersion, first stir for 12 hours, then place in a constant temperature shaker at 37°C for 12 hours, and finally The product was centrifuged, washed with ethanol and deionized water alternately and repeatedly for 3 times, and the product MSNs-PEI was obtained;
[0061] 4) Dissolve 4mg of miRNAs in 15mL of N,N-dimethylformamide (DMF), then add a total of 0.3g of EDC and NHS with a molar ratio of 2.5:1, and stir at 32°C for 2 hours to activate;
[0062] 5) Dispersing the MSNs-PEI prepa...
Embodiment 2
[0069] 1) Disperse 50 mg of surface carboxylated mesoporous silicon (MSNs-COOH) into 50 mL of dimethyl sulfoxide (DMSO), and disperse it evenly by ultrasonication;
[0070] 2) Weigh a total amount of 0.8g of EDC and NHS with a molar ratio of 2.5:1 and add them to the above dispersion, stir at 32°C for 2 hours to activate the carboxyl groups on MSNs-COOH;
[0071] 3) Add 50mL polyethyleneimine (PEI) aqueous solution with a concentration of 80mg / mL dropwise to the MSNs-COOH dispersion with activated carboxyl groups, stir for 12 hours, then put it into a constant temperature shaker at 37°C for 12 hours; finally The product was centrifuged, washed with ethanol and deionized water alternately and repeatedly for 3 times, and the product MSNs-PEI was obtained;
[0072] 4) Dissolve 4mg of miRNAs in 15mL of N,N-dimethylformamide (DMF), then add a total of 0.3g of EDC and NHS with a molar ratio of 2.5:1, and stir at 32°C for 2 hours to activate;
[0073] 5) Dispersing the MSNs-PEI prep...
Embodiment 3
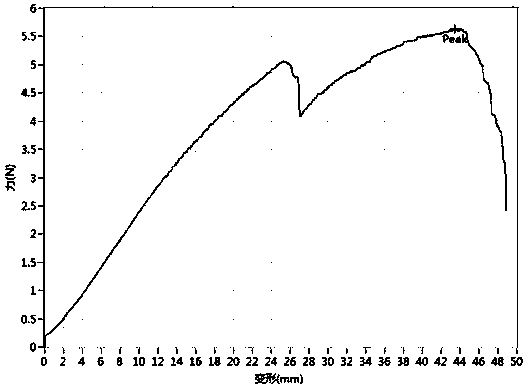
[0080] Test the axial mechanical data of the double-layer artificial blood vessel modified by the mesoporous miRNAs controlled-release nanosphere / heparin compound prepared in Example 1, and the results are shown in the following table:
[0081] Table 1
[0082]
[0083] Test its axial force-deformation diagram as image 3 shown. The first peak point (5.05N, 25.5mm) in the figure is the breaking point of the macroporous layer material obtained by the phase separation of the outer layer, and the second peak point (Peak point) is the breaking point of the inner nanofiber material .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com