Method and application for promoting cells to secrete exosomes
A cell secretion and exosome technology, applied in the field of promoting cell secretion of exosomes, can solve problems such as single function and limited ability to secrete exosomes, achieve high yield, promote tissue repair function, and promote rapid wound repair. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Exosomes produced by cells stimulated with biomaterial activity signals:
[0042] Step 1, prepare bioactive glass (BG) ion extraction solution, respectively add 1g BG powder to 5mL serum-free mesenchymal stem cell culture medium (MSCM) and 5mL serum-free endothelial cell culture medium (ECM), in the cell Incubate in an incubator for 24 hours, collect the supernatant, and filter the filtrate through a 0.22 μm sterile filter to obtain a BG ion extract, which is stored at 4°C for use.
[0043] Step 2, preparing directional electrospinning fiber membrane, dissolving PCL and PDLLA with a mass ratio of 1:1 in DMF and THF with a volume ratio of 4:1 at a mass volume ratio of 4.8%, and using an electrospinning device to 10kv Electrospinning was carried out under the conditions of voltage, 0.02mL / min flow rate and 12cm receiving distance, and the oriented electrospun fiber membrane was received by a high-speed drum rotating at 2000r / min, which was cut into discs with a diameter o...
Embodiment 2
[0052] Verification of the ability of HBMSC-derived exosomes to promote HUVEC angiogenic differentiation in vitro:
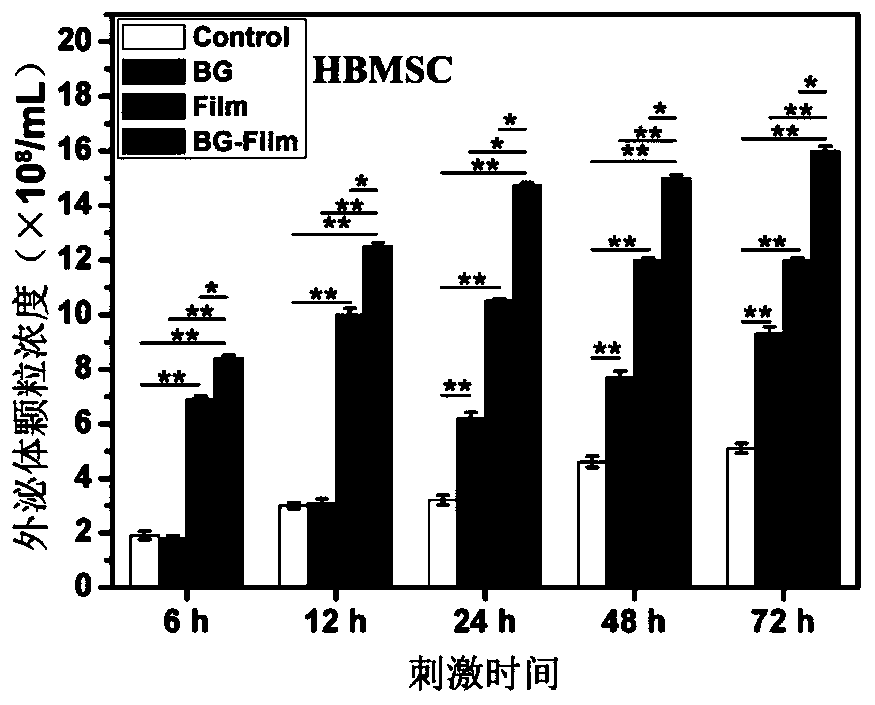
[0053] According to the method shown in Example 1, the exosomes secreted by HBMSCs under BG stimulation for 48 hours and under normal conditions were collected, respectively recorded as HBMSC-BG-exosome and HBMSC-Control-exosome, and stored at 4°C for future use.
[0054] Digest passage HUVEC, according to 4 × 10 4 HUVEC / cm2 cell density were inoculated into sterile 6-well culture plate and 48-well plate covered with cell slides respectively, cultured with complete ECM for 24 hours, washed with PBS three times, and HBMSC-BG- exosome and HBMSC-Control-exosome diluted to 1 × 10 8 pcs / mL, add 2 mL of HBMSC-BG-exosome exosome dilution to each well of the first 6-well plate, add 2 mL of HBMSC-Control-exosome exosome dilution to each well of the second 6-well plate, No exosome dilution solution was added to the third 6-well plate, 0.5 mL of HBMSC-BG-exosome exosome ...
Embodiment 3
[0058] Verification of the ability of HBMSC-derived exosomes to promote HUVEC subcutaneous angiogenesis in vivo:
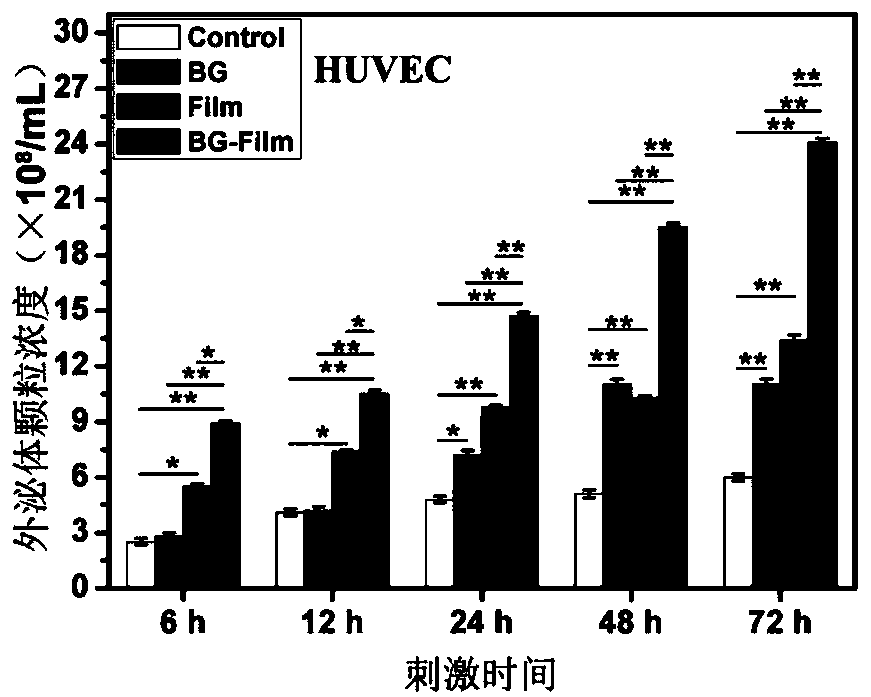
[0059] According to the method shown in Example 1, the exosomes secreted by HBMSCs under BG stimulation for 48 hours and under normal conditions were collected, respectively recorded as HBMSC-BG-exosome and HBMSC-Control-exosome, and stored at 4°C for future use.
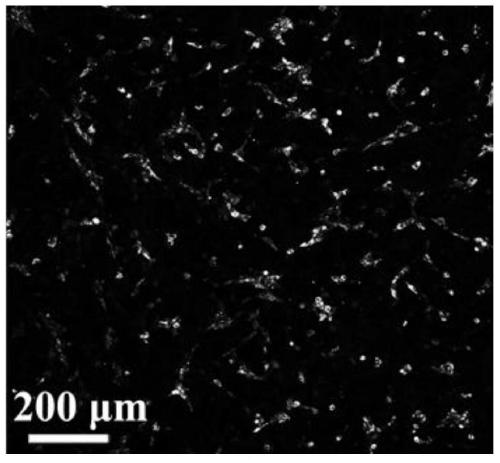
[0060] On the back of three Kunming mice, a small area of hair was shaved with a shaving machine, and the first mouse was injected with 50 μL of particles with a concentration of 1×10 8 / mL PBS solution of HBMSC-BG-exosome, the second injection of 50 μL containing particles with a concentration of 1×10 8 / mL of the PBS solution of HBMSC-Control-exosome, the third mouse was injected with PBS solution without exosomes as the control (Control), and after 1 week, the experimental mice were killed by neck dislocation, and the skin of the exosome injection area was taken, and the The inner tiny blood vesse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com