A lipase mutant with improved catalytic activity and its application
A technology for improving catalytic activity and lipase, which is applied in the field of enzyme engineering and can solve problems such as the inability to meet industrial needs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of lipase mutants
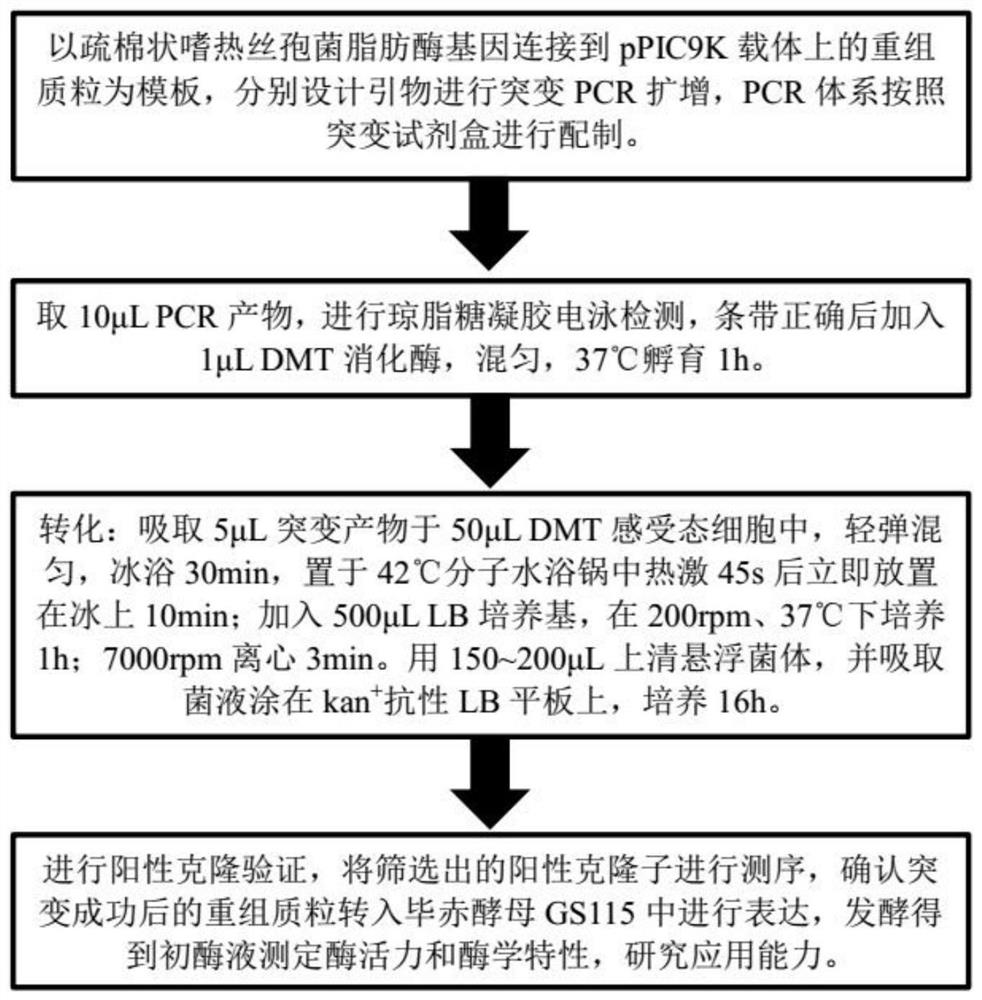
[0048] like figure 1 As shown, using the site-directed mutagenesis kit, using the pPIC9K-TLL recombinant plasmid as a template, and using the following primers, site-directed mutagenesis amplification was performed. After the amplification was completed, 10 μL of PCR product was taken for agarose gel electrophoresis detection, and after verifying that the size of the band was correct, 1 μL of DMT enzyme was added to the PCR product, mixed well, and digested at 37° C. for 1 hour. Then transform: add 3 μL of digested product to 50 μL DMT competent cells, ice bath for 30 min, then heat shock in a molecular water bath at 42 °C for 45 s, ice bath for 2 min, add 500 μL of LLB medium to the product, at 37 °C, 180rpm Incubate in a shaker for 1 hour, and finally take 200 μL of bacterial solution and apply it to kan + Resistant LB dishes were incubated overnight in a 37°C incubator. On the second day, a single colony on the plate was r...
Embodiment 2
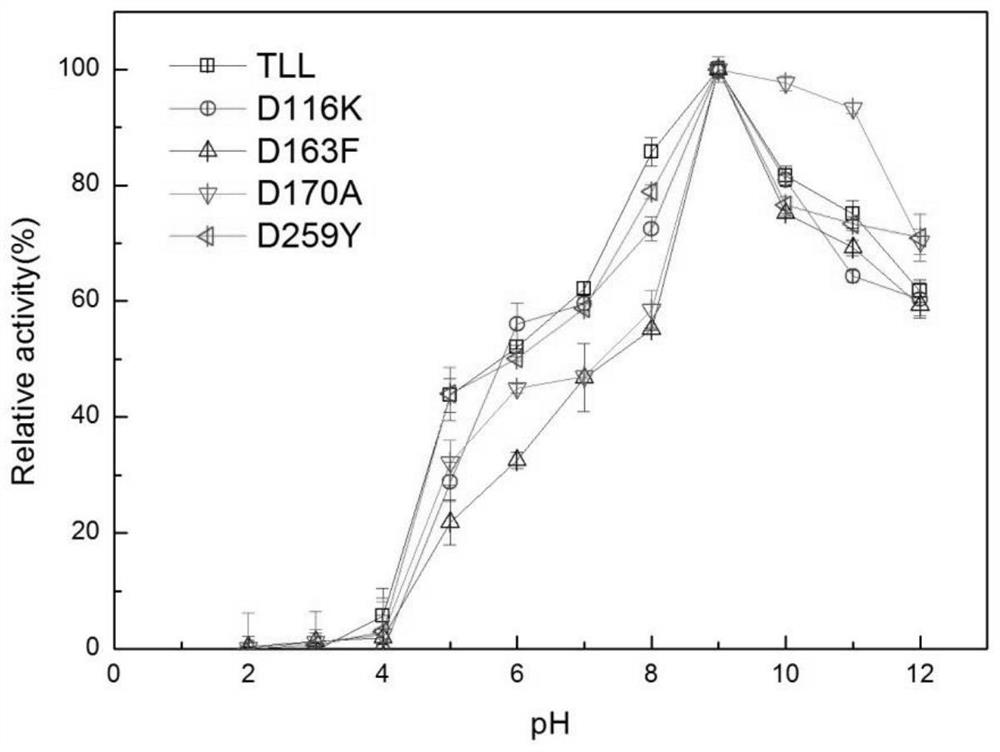
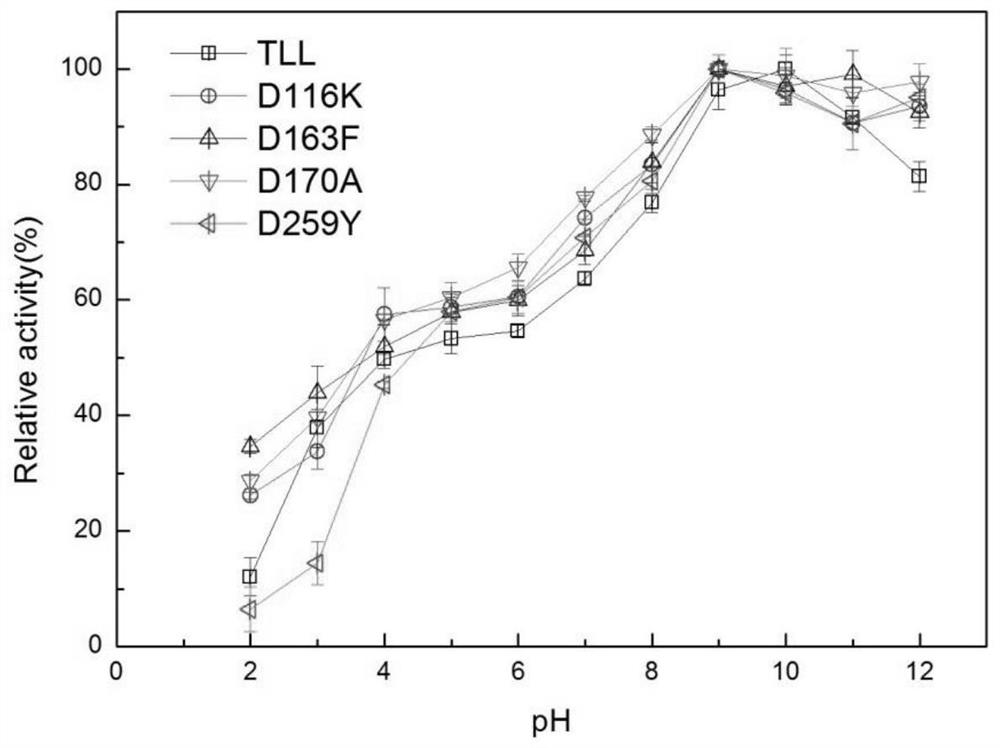
[0057] Example 2 Determination of enzyme activity and enzymatic properties of lipase mutants
[0058] 1. Determination of enzyme activity of lipase mutants
[0059] The unit of enzyme activity is defined as: the amount of enzyme required to hydrolyze the substrate p-NP per minute under certain conditions to generate 1 μmoL of p-nitrophenol is one unit of enzyme activity, expressed as U. p-Nitrophenol method: pipette 420 μL of 50 mM Tris-HCl buffer at pH 9.0 into a centrifuge tube, then add 30 μL of 10 mM substrate p-NP, mix well, warm up at 37 °C for 2 min, and then add the diluted enzyme Add 50 μL of 10% SDS to stop the reaction, and finally add 500 μL of 0.5M sodium carbonate to develop color, and measure its OD value with a microplate reader at a wavelength of 405 nm. The enzyme activity assay results of the mutants are shown in Table 1: the enzyme activities of the mutants D116K, D163F, D170A, and D259Y were increased by 35.5%, 17.0%, and 44.2%, respectively, compared wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com