Method for preparing tranexamic acid
A technology of tranexamic acid and methanol is applied in the field of preparation of tranexamic acid, and achieves the effects of high yield, simple operation, cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
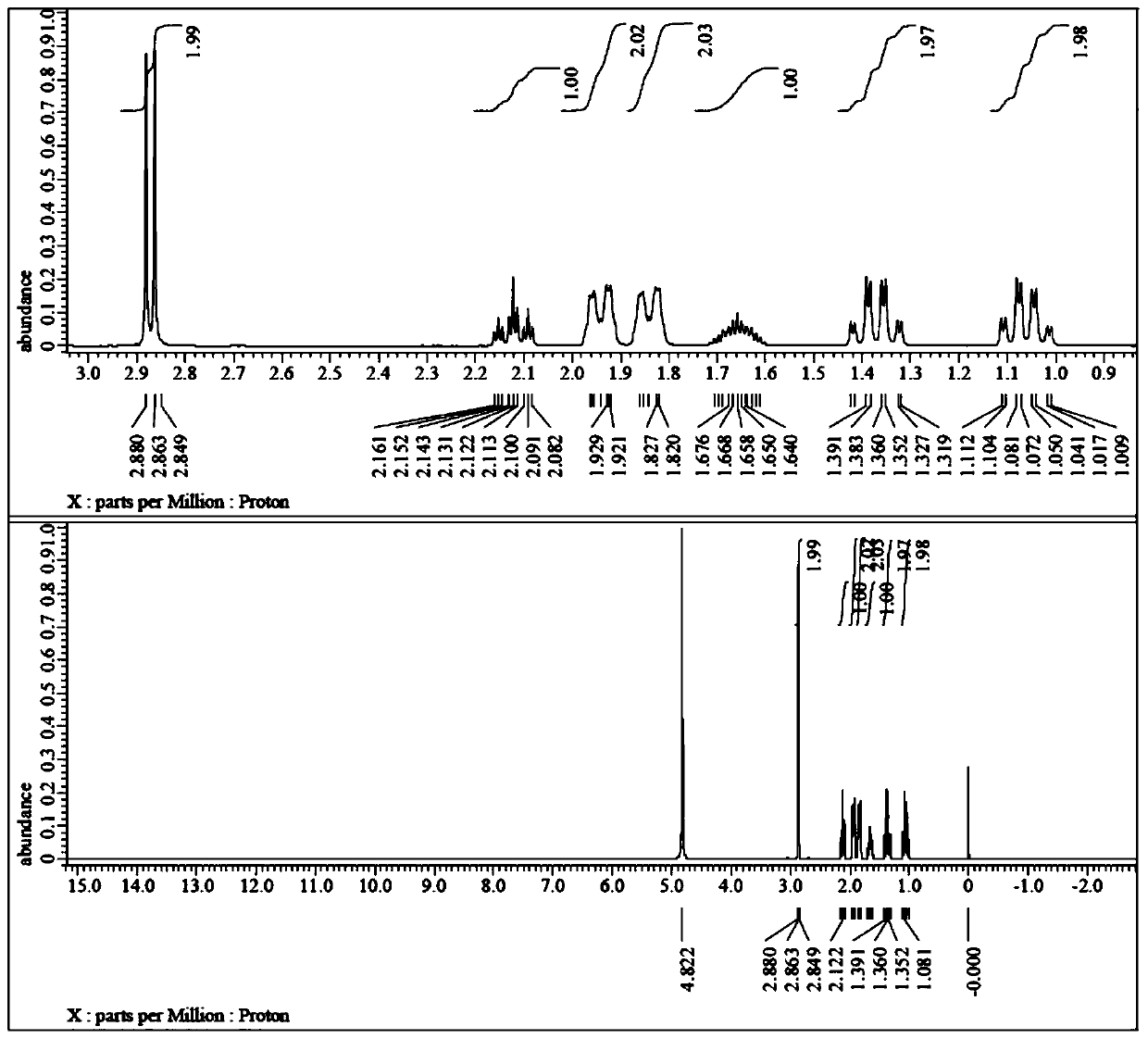
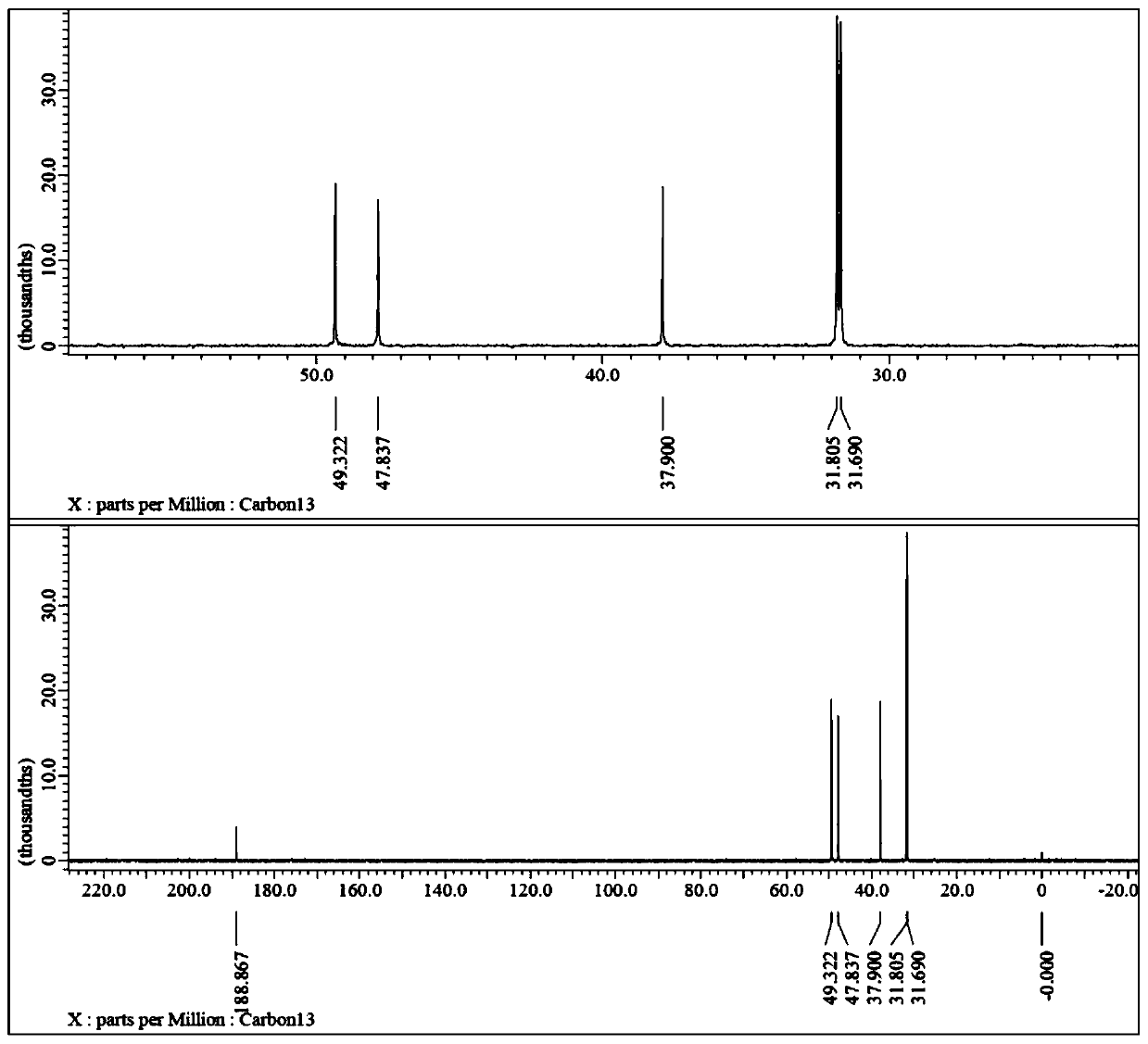
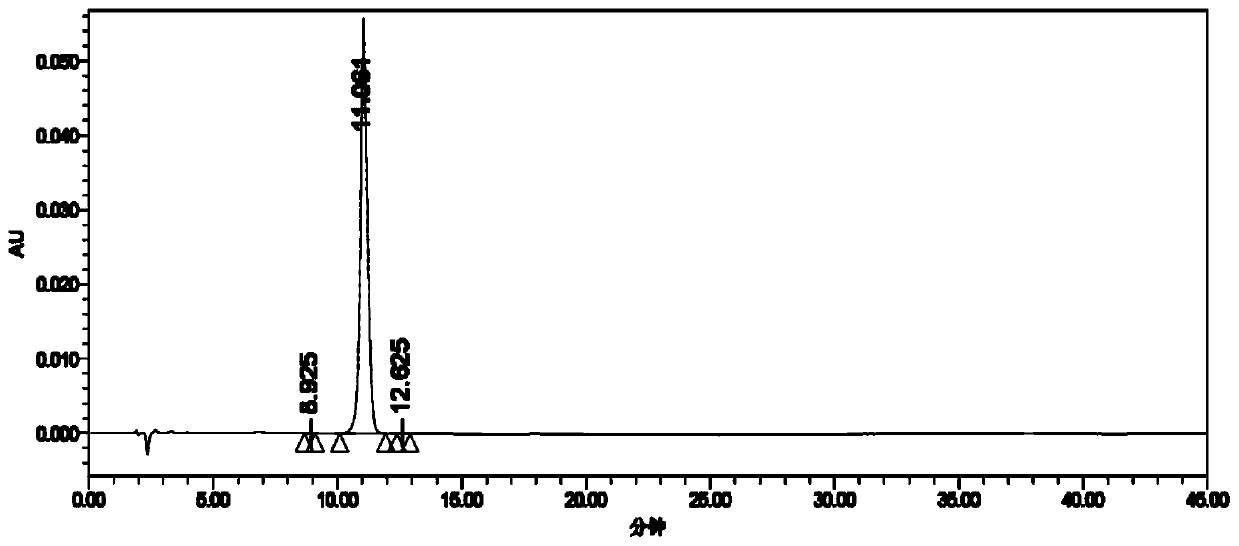
[0048] 1) Add 288.4g (2mol) of 1,4-cyclohexanedimethanol (cis-trans mixed) and 608.4g of hydrochloric acid (6mol) with a mass fraction of 36% to the reactor, and stir the reaction at 85°C for 12h. After the reaction, cool to room temperature, add 300g of toluene for extraction, and wash with water until neutral. Concentrated under reduced pressure to obtain 257.6g of 4-chloromethylcyclohexylmethanol (cis-trans mixed), GC purity 91.7%, yield 79.2%;
[0049] 2) Add 81.3g (0.5mol) 4-chloromethylcyclohexylmethanol (cis-trans mixed) and 500g trifluoroacetic acid to the reactor, add 69g (1mol) sodium nitrite under stirring at 0°C in an oxygen atmosphere, Then rise to 25 ℃ for 6h. Add 300g of dichloromethane after concentrating under reduced pressure, adjust to neutral with 5% sodium bicarbonate solution, separate the water phase, and concentrate the organic phase under reduced pressure to obtain 86.6g of 4-chloromethylcyclohexyl formic acid (cis and trans mixed), GC Purity 90.8%, ...
Embodiment 2
[0052] 1) Add 288.4g (2mol) trans-1,4-cyclohexanedimethanol and 608.4g hydrochloric acid (6mol) with a mass fraction of 36% to the reactor, and stir at 90°C for 10h. After the reaction, cool to room temperature, add 300g of toluene for extraction, and wash with water until neutral. Concentrated under reduced pressure to obtain 259.3 g of trans-4-chloromethylcyclohexylmethanol with a GC purity of 92.1% and a yield of 79.7%;
[0053] 2) Add 81.3g (0.5mol) trans-4-chloromethylcyclohexylmethanol and 500g trifluoroacetic acid to the reactor, add 69g (1mol) under stirring at 0°C in an air atmosphere with an oxygen content of 21% Sodium nitrite, then rose to 30 ° C for 5h. After concentration under reduced pressure, 300 g of dichloromethane was added, adjusted to neutral with 5% sodium bicarbonate solution, the water phase was separated, and the organic phase was concentrated under reduced pressure to obtain 86.8 g of trans-4-chloromethylcyclohexylcarboxylic acid, GC purity 91.5%, ...
Embodiment 3
[0058] 1) Add 288.4g (2mol) of 1,4-cyclohexanedimethanol (cis-trans mixed) and 1088.6g of hydriodic acid (4mol) with a mass fraction of 47% into the reactor, and stir the reaction at 80°C for 8h. After the reaction, cool to room temperature, add 500g of toluene for extraction, and wash with water until neutral. Concentrate under reduced pressure to obtain 385.7 g of 4-iodomethylcyclohexylmethanol (cis-trans mixed), GC purity 91.2%, yield 75.9%.
[0059] 2) Add 127.1g (0.5mol) 4-iodomethylcyclohexylmethanol (cis-trans mixed) and 500g trifluoroacetic acid into the reactor, add 69g (1mol) sodium nitrite under stirring at 0°C in an oxygen atmosphere, Then it was raised to 25°C for 5h. After concentrating under reduced pressure, add 300g of dichloromethane, adjust to neutral with 5% sodium bicarbonate solution, separate the water phase, and concentrate the organic phase under reduced pressure to obtain 130.9g of 4-iodomethylcyclohexylcarboxylic acid (cis and reverse mixed), GC pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com