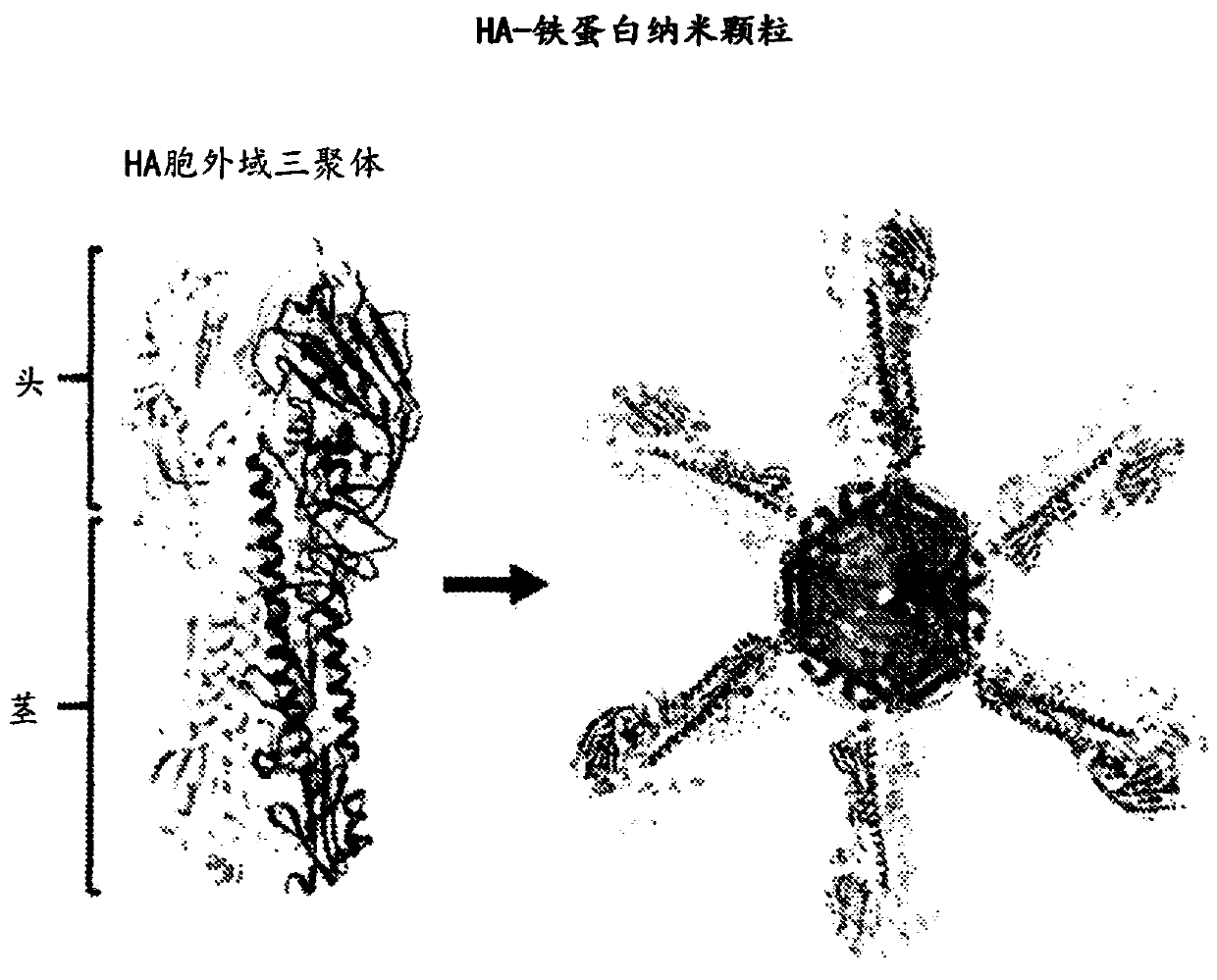
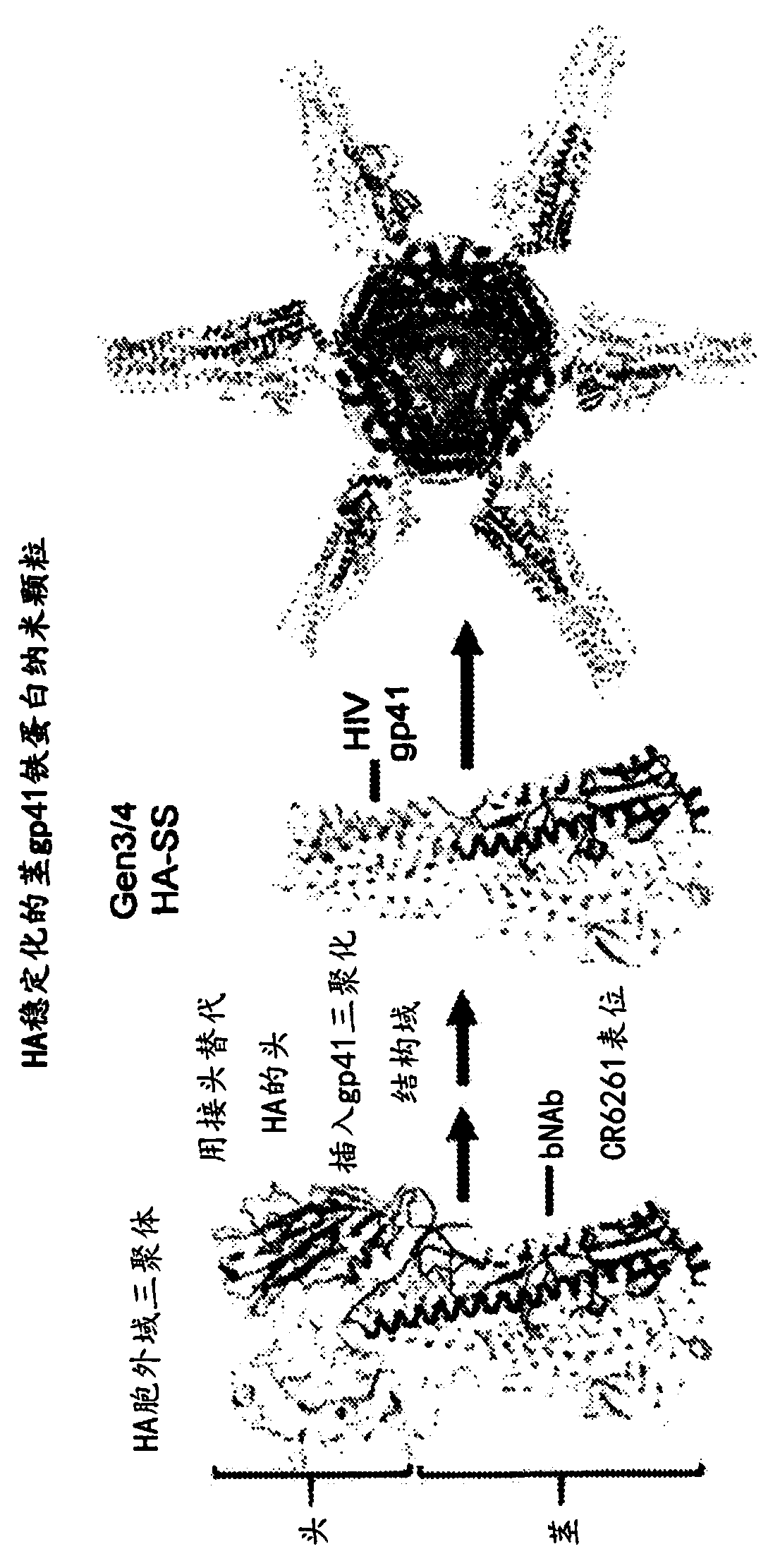
Stabilized group 2 influenza hemagglutinin stem region trimers and uses thereof
A technology for hemagglutinin and influenza, applied to medical preparations containing active ingredients, antibody medical ingredients, viruses/bacteriophages, etc., can solve the problem of limited ability to induce protective neutralizing antibody titers, and is unlikely to significantly improve protection Issues such as potency and breadth of sexual immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0268] This example characterizes the properties and activities of five Group 2 HA nanoparticle H10 variants designed using the parameters and methods disclosed in this application. All these variants are based on the human A / Jiangxi / IPB13 / 2013 (H10N8) strain. A nucleic acid molecule encoding an H10 variant is introduced into Expi293 cells, and the cells are cultured under conditions suitable for expression of the encoded variant protein. Expressed nanoparticles were purified from cell culture supernatants using lectin affinity chromatography followed by size exclusion chromatography (SEC). Chromatograms of purified nanoparticles as Figures 32A-32E shown.
[0269] The purified nanoparticles were analyzed using negative-stain electron microscopy, which revealed the formation of individual nanoparticles with HA stems protruding outwards in a periodic arrangement. Representative SEM images of each variant are shown in Figure 33.
[0270] The antigenicity of H10ssF variants w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com