Synthesis method of 9H-xanthene/ thioxanthene compound
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve problems such as difficult industrial production, unfavorable energy conservation and environmental protection, and achieve the effects of less waste discharge, high yield and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthetic method of 9-(2,4-dimethoxyphenyl)-9H-oxanthene.
[0047] Take a 15mL pressure-resistant reaction tube, add DDQ 45mg, xanthene 36mg, 1,3-dimethoxybenzene 83mg, molecular sieve 100mg, acetonitrile 2mL, open the reaction, and stir at room temperature for 12h. After the reaction, 10 mL of ethyl acetate was added to quench the reaction, washed with 10 mL of brine, the organic phase was separated, the aqueous phase was extracted 3 times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 9-(2,4-dimethyl Oxyphenyl)-9H-xanthene pure product 50mg, productive rate 83%, the molecular structural formula of gained 9-(2,4-dimethoxyphenyl)-9H-xanthene is
[0048]
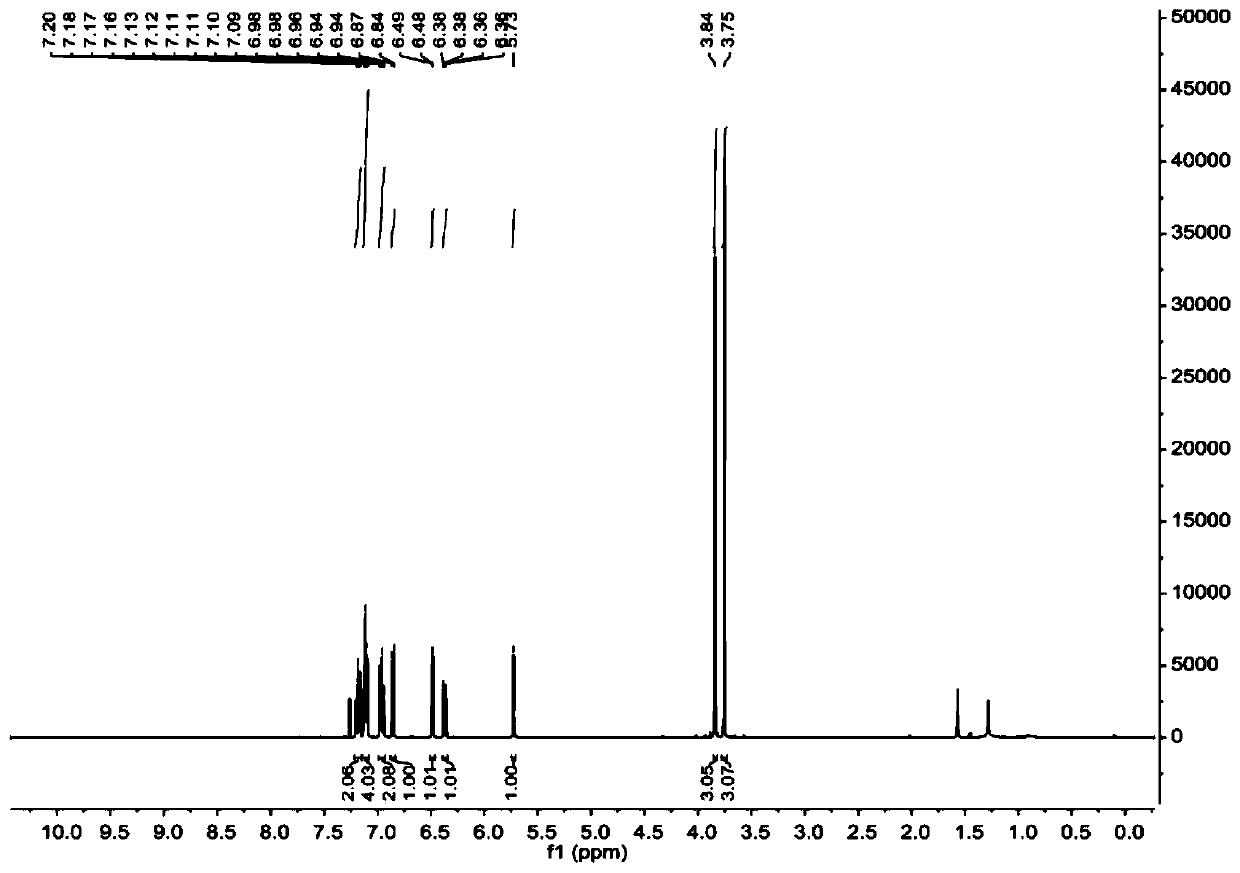
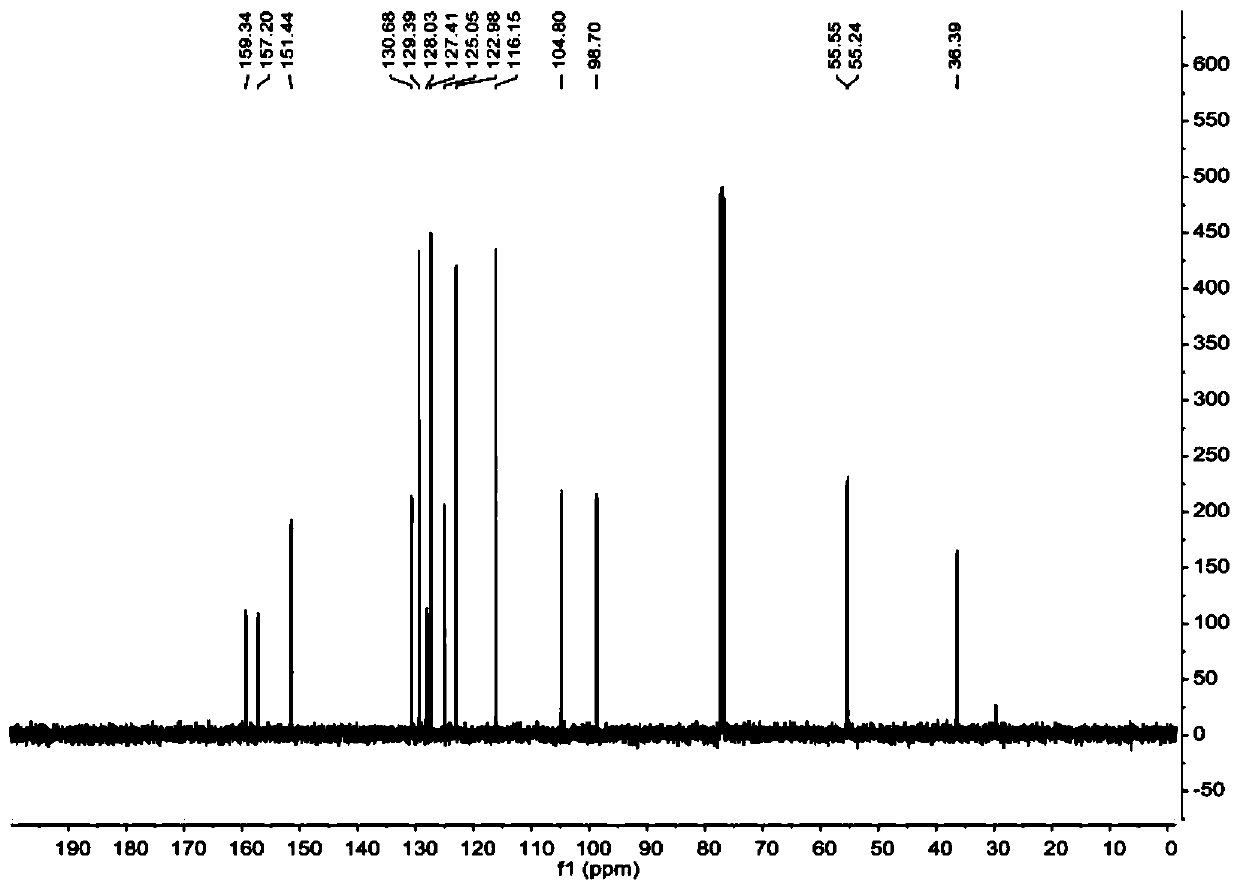
[0049] figure 1 and figure 2 These are the NMR H spectrum and NMR C spectrum of 9-(2,4-dimethoxyphenyl)-9H-xanthene prepared in this example, respectively. figure 1 The chemical shifts of peaks in the hydrogen spectrum 1H NMR (400MHz, Chloroform-d) δ7.22-7....
Embodiment 2
[0051] Synthetic method of 9-(2,4,6-trimethoxyphenyl)-9H-oxygen / thioxanthene.
[0052]Take a 15mL pressure-resistant reaction tube, add DDQ 45mg, oxygen / thioxanthene 36mg, 1,3,5-trimethoxybenzene 101mg, molecular sieve 100mg, acetonitrile 2mL, open the reaction, and stir at room temperature for 12h. After the reaction was completed, 10 mL of ethyl acetate was added to quench the reaction, and 10 mL of brine was added for washing. The organic phase was separated, and the aqueous phase was extracted 3 times with ethyl acetate. The organic phases were combined and separated by column chromatography to obtain 9-(2,4,6- The pure product of trimethoxyphenyl)-9H-oxygen / thioxanthene was 65 mg, and the yield was 99%.
Embodiment 3
[0054] Synthetic method of 9-(2,4-dimethoxyphenyl)-9H-thioxanthene.
[0055] Take a 15mL pressure-resistant reaction tube, add 45mg of DDQ, 39mg of thioxanthene, 83mg of 1,3-dimethoxybenzene, 100mg of molecular sieves, 2mL of acetonitrile, open the reaction, and stir at room temperature for 12h. After the reaction, 10 mL of ethyl acetate was added to quench the reaction, washed with 10 mL of brine, the organic phase was separated, the aqueous phase was extracted 3 times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 9-(2,4-dimethyl The pure product of oxyphenyl)-9H-thioxanthene was 42mg, and the yield was 70%. The molecular structural formula of gained 9-(2,4-dimethoxyphenyl)-9H-thioxanthene is
[0056]
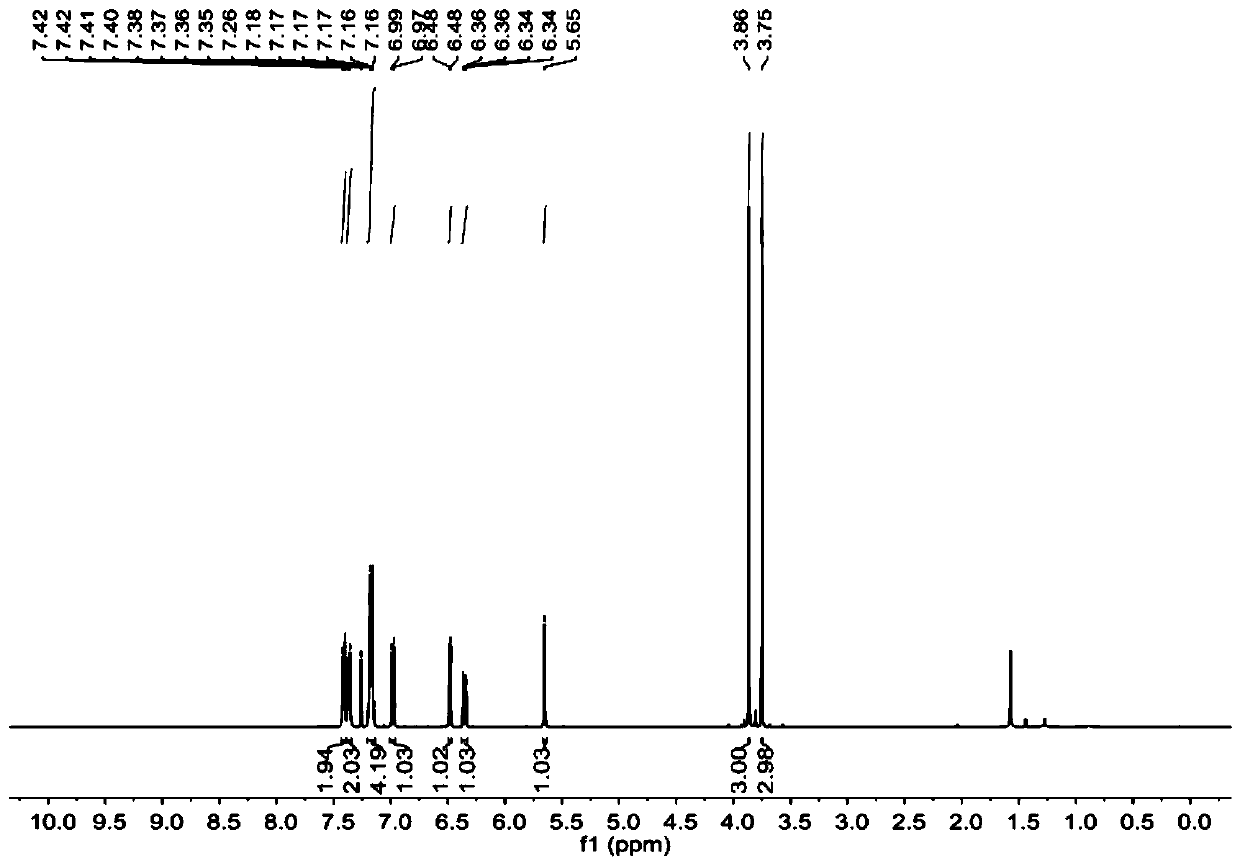
[0057] image 3 and Figure 4 These are the NMR H spectrum and NMR C spectrum of 9-(2,4-dimethoxyphenyl)-9H-thiaxanthene prepared in this example, respectively. image 3 The chemical shifts of the peaks in the hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com