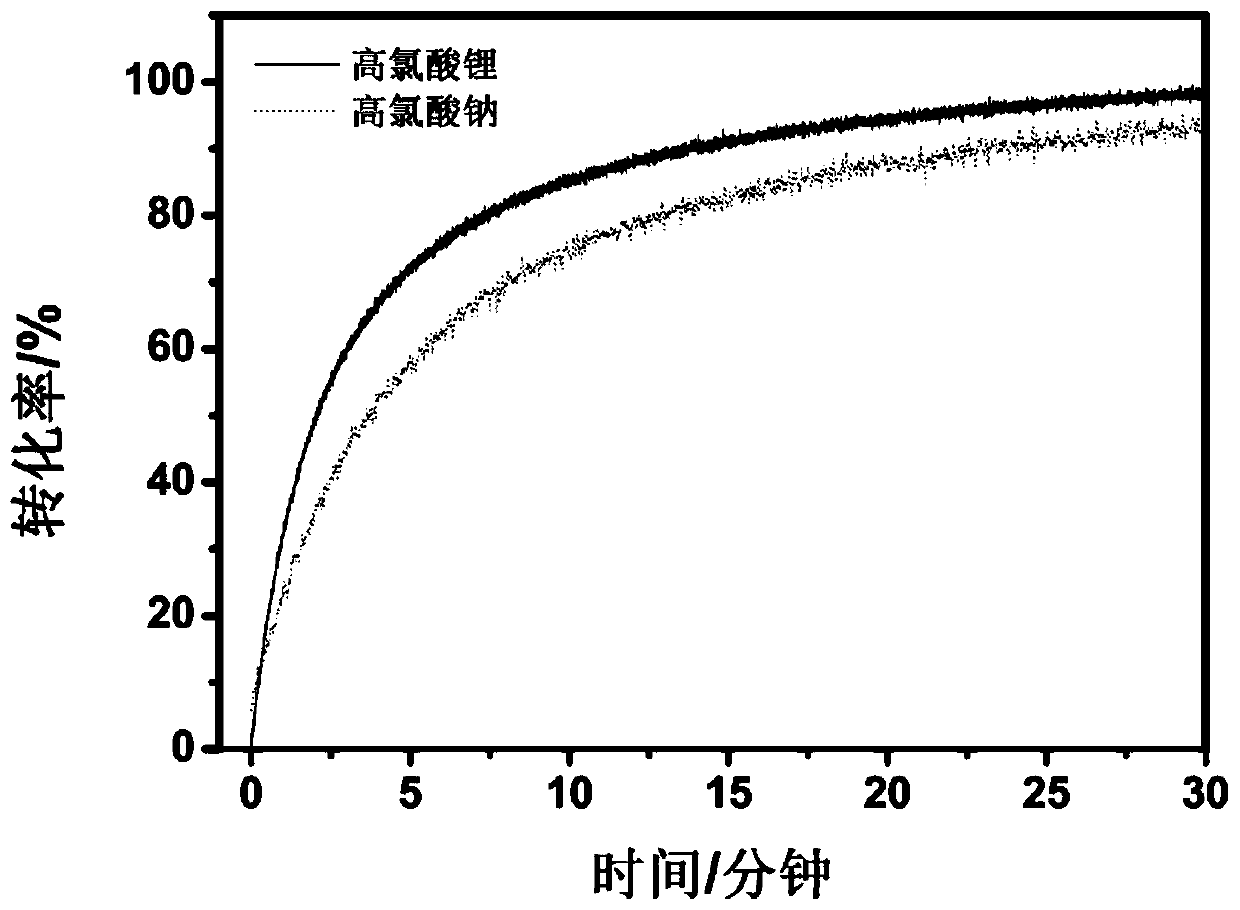
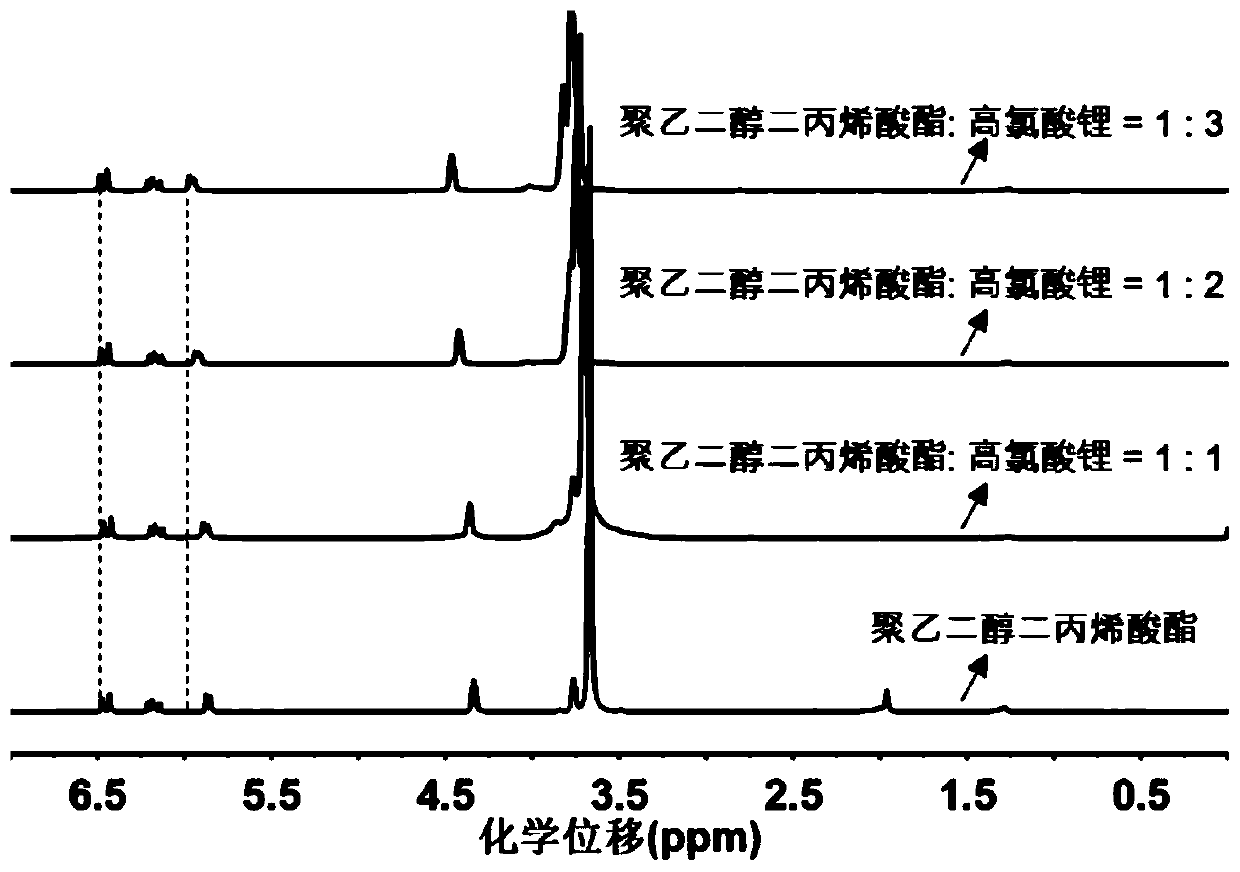
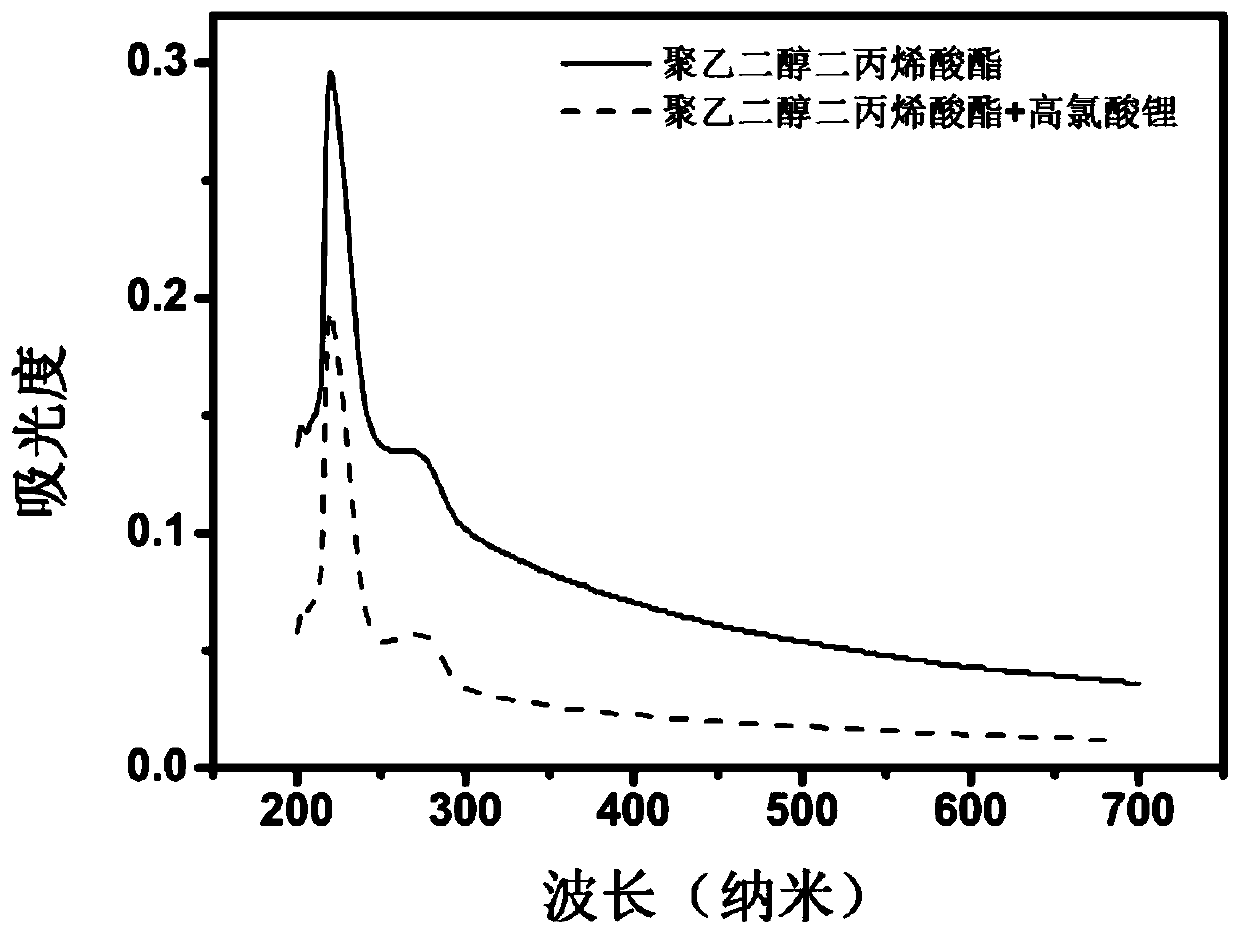
Preparing method and application of polymer electrolyte
A technology of reaction and reactants, which is applied in the field of polymer electrolytes, can solve the problems of reducing the electron cloud density of electron-deficient double bonds, high crystallinity of solid-state electrolytes, complex production processes, etc., to overcome the low reaction rate and conversion rate, reduce the Degree of damage and cost of response, avoiding expensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention also provides a method for preparing a polymer electrolyte, using the first reactant containing mercapto terminal and the second reactant containing acrylate terminal at both ends as raw materials, under the action of the main catalyst and the co-catalyst, the mercapto- Michael addition reaction to prepare a polymer electrolyte; where:
[0056] The first reactant contains at least three mercapto groups;
[0057] The main catalyst is used to capture the proton on the mercapto group of the first reactant, and then form a thiol anion, which is used to attack the electron-deficient double bond in the second reactant;
[0058] The cocatalyst is a metal salt; the cation of the metal salt undergoes a complex reaction with the second reactant, reduces the electron cloud density of the double bond of the acrylate, and promotes the attack of the thiol anion on the double bond of the acrylate. The bond undergoes mercapto-Michael addition reaction, which incr...
Embodiment 1
[0071] A method for metal salt-promoted mercapto-Michael addition reaction, carried out according to the following steps:
[0072] 1-hexanethiol, 2-propenoic acid-2-methoxyethyl ester and lithium bistrifluoromethanesulfonylimide were prepared as reaction components, 1-hexanethiol, 2-propenoic acid-2-methoxyethyl The molar ratio of ester to lithium bistrifluoromethanesulfonylimide is 5:5:1. The preparation process is as follows: put 1.41 ml of 1-hexanethiol, 1.29 ml of 2-propenoic acid-2-methoxyethyl ester and 0.574 g of lithium bistrifluoromethanesulfonylimide into a round bottom flask, and after magnetic stirring for 4 hours, , to obtain a reaction mixture.
[0073] Take 70 microliters of purified triethylamine and add it to the reaction mixture in the round bottom flask. The molar ratio of triethylamine to the sulfhydryl group of 1-hexanethiol is 1:20. After 10 seconds of magnetic stirring, quickly take out a small amount of the reaction mixture. , added dropwise between t...
Embodiment 2
[0075] A method for metal salt-promoted mercapto-Michael addition reaction, carried out according to the following steps:
[0076] 1-hexanethiol, 2-propenoic acid-2-methoxyethyl ester and lithium bistrifluoromethanesulfonylimide were prepared as reaction components, 1-hexanethiol, 2-propenoic acid-2-methoxyethyl The molar ratio of ester to lithium bistrifluoromethanesulfonylimide is 100:100:1. The preparation process is as follows: put 1.41 ml of 1-hexanethiol, 1.29 ml of 2-propenoic acid-2-methoxyethyl ester and 0.029 g of lithium bistrifluoromethanesulfonimide into a round bottom flask, and after magnetic stirring for 4 hours, , to obtain a reaction mixture.
[0077] Take 278 microliters of purified triethylamine and add it to the reaction mixture in the round bottom flask. The molar ratio of triethylamine to the sulfhydryl group of 1-hexanethiol is 1:5. After magnetic stirring for 10 seconds, quickly take out a small amount of the reaction mixture , added dropwise between...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com