Method for preparing high-purity lithium fluoride
A fluorinated, high-purity technology, applied in lithium halide and other directions, can solve the problems of production restrictions and high quality requirements, and achieve the effects of low raw material cost, high product conversion rate and uniform particle size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
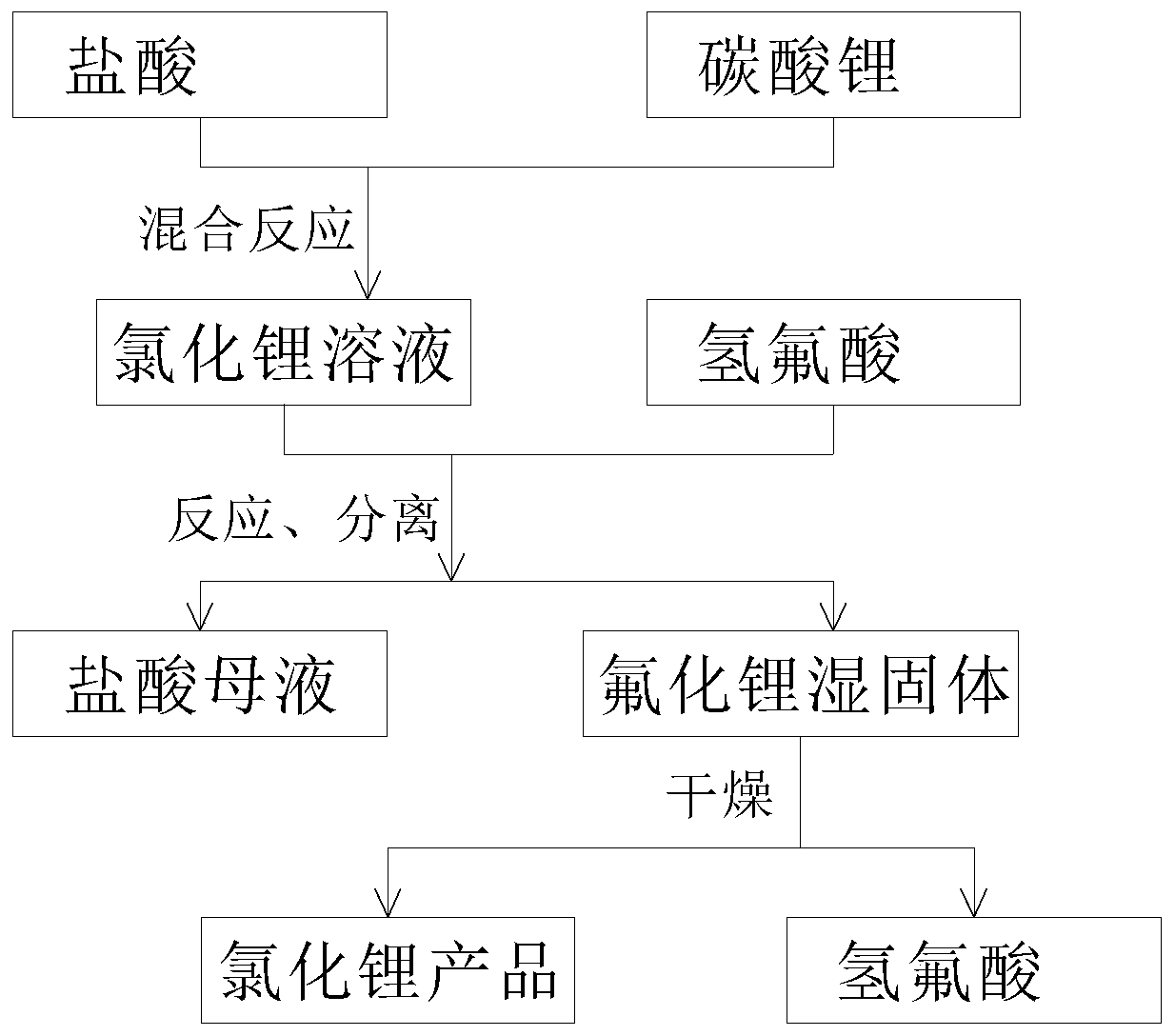
[0030] 1) Weigh 28g of lithium carbonate powder and add it to a 200ml polytetrafluoroethylene beaker, slowly add 90ml of 36% hydrochloric acid under stirring, and stir until lithium carbonate is completely dissolved;
[0031] 2) Heat the solution obtained by the above reaction to 80° C., slowly add 50 ml of hydrofluoric acid with a mass percent concentration of 40% under stirring, and continue to stir and react for 1.5 hours to obtain a mixed solution containing lithium fluoride precipitate;
[0032] 3) The above-mentioned mixed solution was left to stand for 1 hour, and then separated by a centrifuge to obtain a fluoride physical precipitate and a mother liquor of hydrochloric acid, and the mother liquor of hydrochloric acid can be recovered as a raw material for the reaction with lithium carbonate in step 1).
[0033] 4) Wash the fluoride precipitate obtained in step 3) with 75°C deionized water for 5 times, separate it with a centrifuge, put it in a vacuum drying oven, and d...
Embodiment 2
[0035] 1) Weigh 28g of lithium carbonate powder and add it to a 200ml polytetrafluoroethylene beaker, slowly add 90ml of 36% hydrochloric acid under stirring, and stir until lithium carbonate is completely dissolved;
[0036] 2) Heat the solution obtained by the above reaction to 90° C., slowly add 150 ml of hydrofluoric acid with a mass percent concentration of 10% under stirring, and continue to stir and react for 2 hours to obtain a mixed solution containing lithium fluoride precipitate;
[0037] 3) The above-mentioned mixed solution was left to stand for 1 hour, and then separated by a centrifuge to obtain a fluoride physical precipitate and a mother liquor of hydrochloric acid, and the mother liquor of hydrochloric acid can be recovered as a raw material for the reaction with lithium carbonate in step 1).
[0038] 4) Wash the fluoride precipitate obtained in step 3) with 75°C deionized water for 5 times, separate it with a centrifuge, put it in a vacuum drying oven, and dr...
Embodiment 3
[0040]1) Weigh 28g of lithium carbonate powder and add it to a 200ml polytetrafluoroethylene beaker, slowly add 90ml of 36% hydrochloric acid under stirring, and stir until lithium carbonate is completely dissolved;
[0041] 2) Heat the solution obtained by the above reaction to 70° C., slowly add 20 ml of hydrofluoric acid with a mass percentage concentration of 99.9% under stirring, and continue stirring for 0.5 hours to obtain a mixed solution containing lithium fluoride precipitate;
[0042] 3) The above-mentioned mixed solution was left to stand for 1 hour, and then separated by a centrifuge to obtain a fluoride physical precipitate and a mother liquor of hydrochloric acid, and the mother liquor of hydrochloric acid can be recovered as a raw material for the reaction with lithium carbonate in step 1).
[0043] 4) Wash the fluoride precipitate obtained in step 3) with 75°C deionized water for 5 times, separate it with a centrifuge, put it in a vacuum drying oven, and dry it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com