Primer, probe and reagent kit for predicting liver cancer susceptibility of patient suffering from hepatitis B
A susceptibility, kit technology, applied in biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc., can solve the problems of reduced HBeAg synthesis and secretion, inhibition of HBeAg synthesis, abnormal HBeAg expression, etc. Easy to judge, high specificity, accurate and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A test kit for predicting susceptibility to liver cancer in patients with hepatitis B, comprising the components shown in Table 3;
[0045] table 3
[0046] serial number components Element quantity Specification 1 PCR reaction solution 1 Primers, probes, Taq enzymes, dNTPs, UNG enzymes, water 1 960μl / tube 2 PCR reaction solution 2 Primers, probes, Taq enzymes, dNTPs, UNG enzymes, water 1 960μl / tube 3 Positive quality control 1 Serum samples of inactivated HBV1896A 1 1.6mL / tube 4 Positive quality control 2 Serum samples of inactivated HBV1762T, 1764A 1 1.6mL / tube 5 Positive Control 3 Serum samples of inactivated HBV1762T, 1764G types 1 1.6mL / tube 6 Positive Control 4 Serum samples of inactivated HBV1762A, 1764A 1 1.6mL / tube 7 negative control Sterilized purified water 1 1.6mL / tube
[0047] Wherein, the PCR reaction solution 1 includes primers and probes for detecting G1896A...
Embodiment 2
[0052] 1. Sample DNA extraction
[0053] Use a commercial viral genome extraction kit to extract sample DNA, and positive quality control products need to participate in the extraction and preparation of HBV genomic DNA.
[0054] 2. PCR reaction system preparation and sample addition
[0055] Prepare PCR reaction system copies n according to the number of samples (n = number of samples + positive control × 4 + negative control), and fill each fluorescent quantitative PCR tube with 20 μL DNA template (DNA sample, negative control, positive control) of the PCR reaction system 10 μL, mixed well and centrifuged briefly, ready to go on the machine.
[0056] Using the kit of Example 1, prepare n tubes of PCR reaction system 1-2 according to Table 4.
[0057] Table 4
[0058]
[0059] 3. PCR amplification detection
[0060] Put the above fluorescent quantitative PCR tube into the fluorescent PCR instrument, and the program settings are shown in Table 5:
[0061] table 5
[0...
Embodiment 3
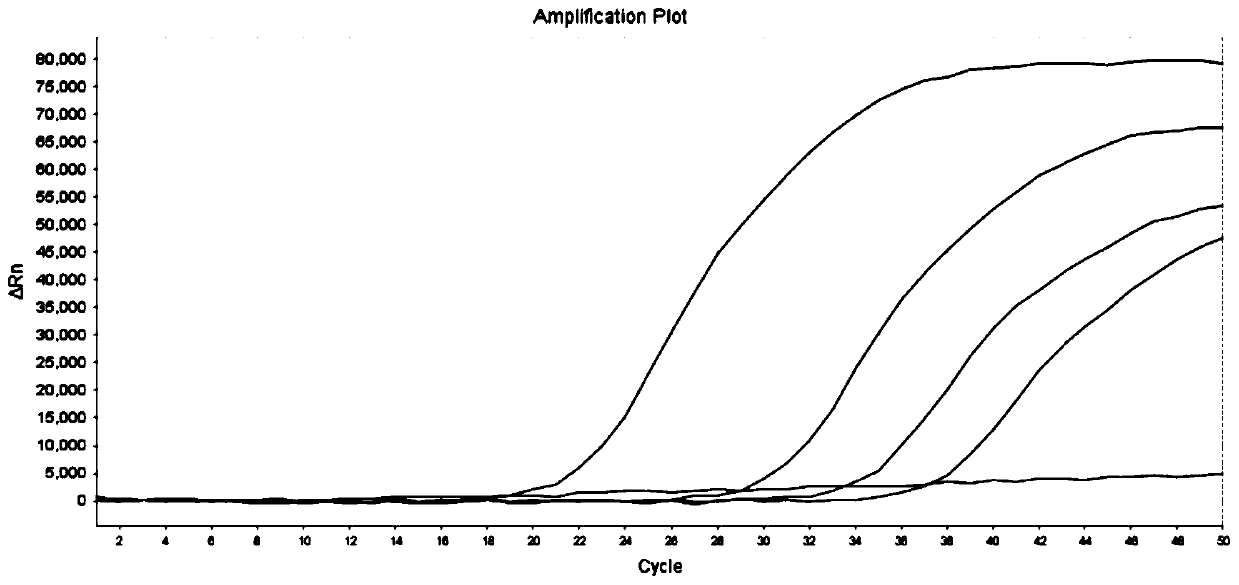
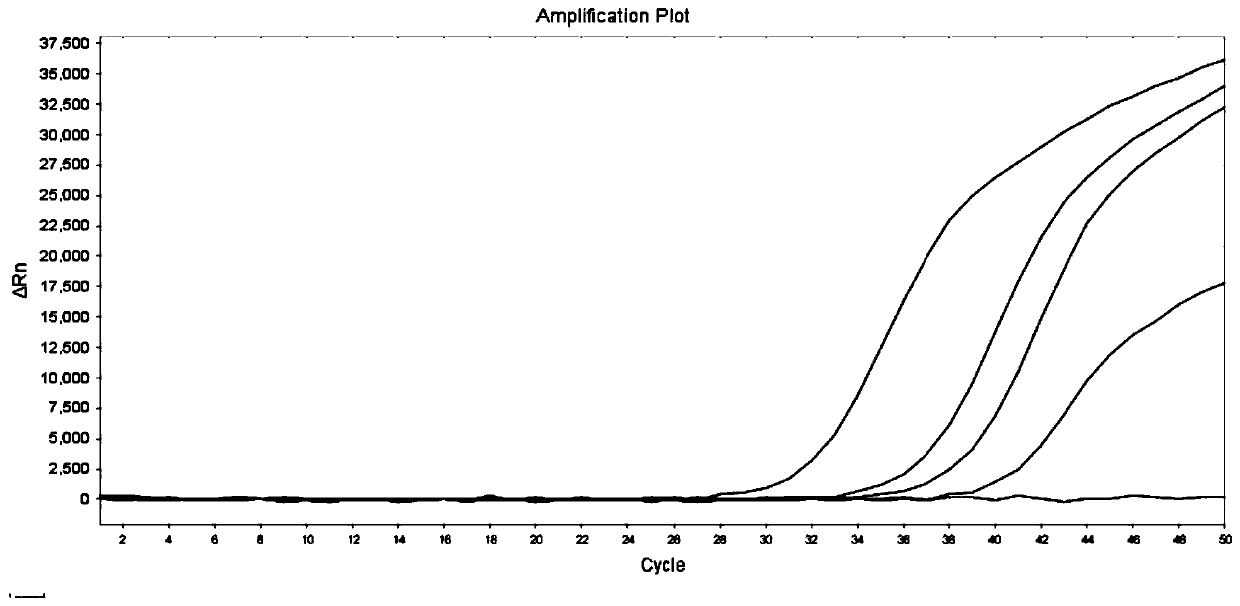
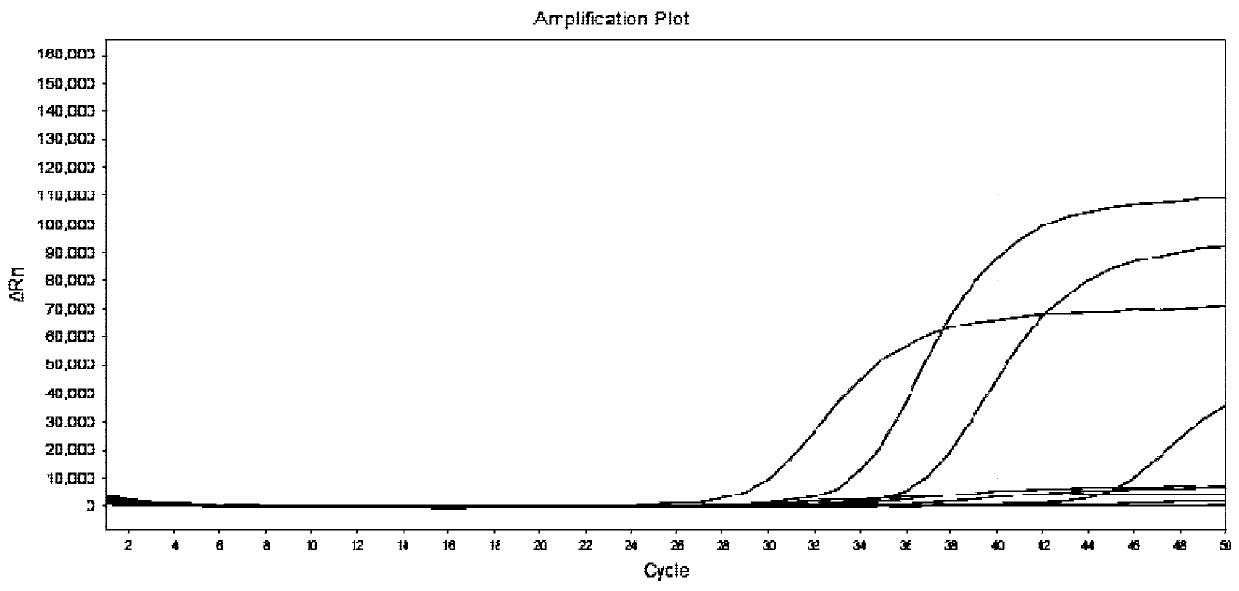
[0072] The method of Example 2 was used to test 18 samples, and the test results are shown in Table 7. Compared with the sequencing results, the consistency was 100%.
[0073] Table 7
[0074]
[0075]
[0076] In summary, the advantages of the primers and probes provided by the present invention are: using MGB modification to improve detection specificity, the accuracy of detection results and sequencing results is as high as 100%; the wild type and mutant type of the site can be detected at the same time; The judgment result of the ΔCt value method is easier to judge, and the result can be accurately typed by the typing software, and can also be confirmed and checked by the fluorescent quantitative PCR curve, and the result is accurate and reliable; Satisfies the needs of gene detection with low virus concentration in the market; not limited by the number of samples, the number of test samples can be allocated, when the number of samples is small, there is no need to s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com