A kind of flavone fluorescent dye and its preparation method and application
A technology of fluorescent dyes and flavonoids, applied in the field of fluorescent sensing, can solve the problems of poor photostability, inability to observe with long-term bioluminescent imaging, and easy photobleaching, etc., and achieve good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
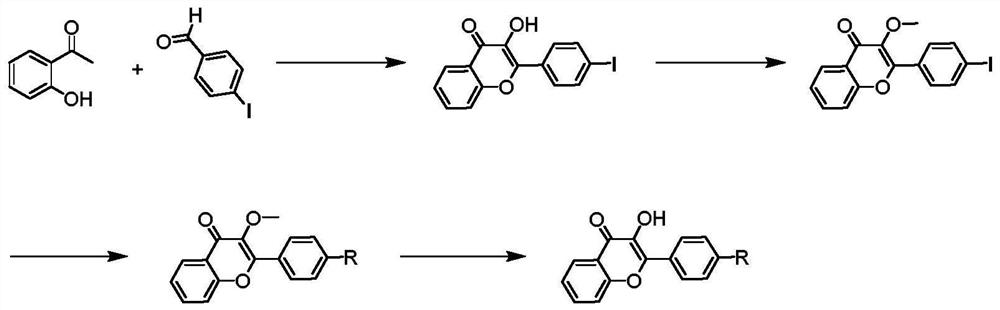
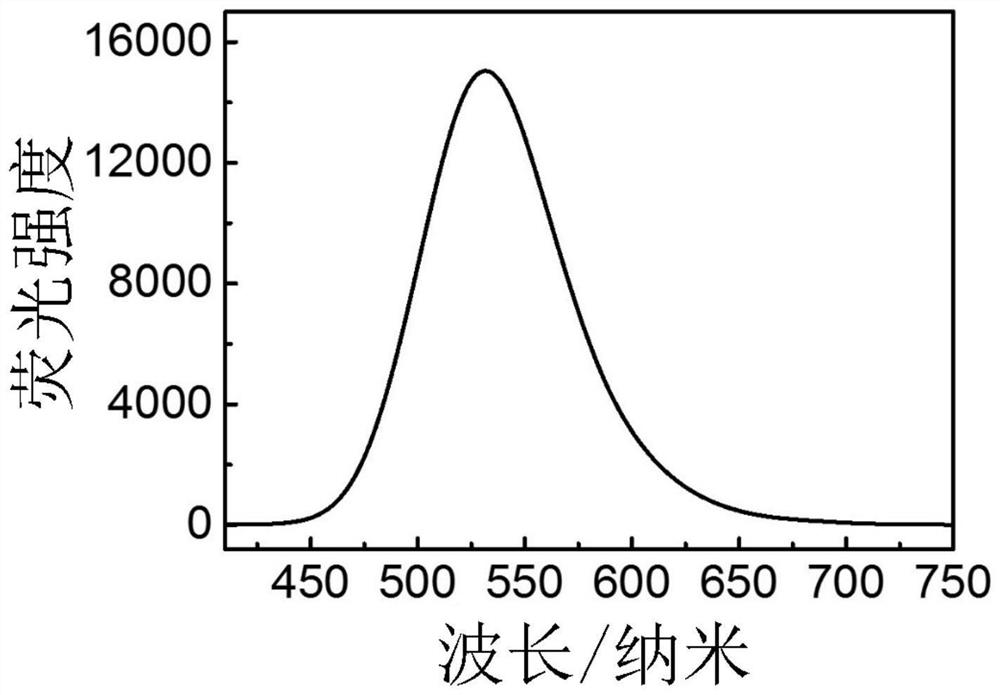
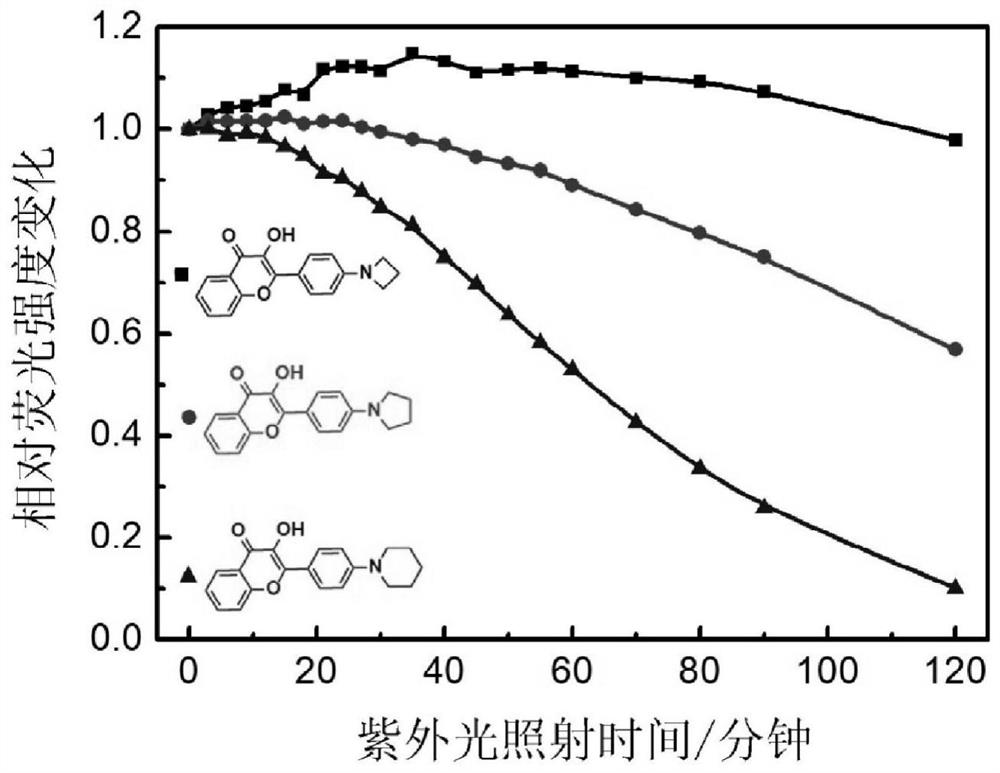
[0067] Preparation of flavonoid fluorescent dye 2-(4-(cyclobutylamino)phenyl)-3-hydroxy-4hydro-chromen-4-one (APHC)
[0068] Add 10 millimoles of o-hydroxyacetophenone and 10 millimoles of p-iodobenzaldehyde into the reactor, add 30 milliliters of ethanol to dissolve, then add 30 milliliters of sodium hydroxide aqueous solution (mass concentration is 20%), and react at 70 degrees Celsius for 12 After one hour, the reaction system was placed in ice-water mixture to cool, and 10 mL of hydrogen peroxide (mass concentration was 30%) was added. After continuing the reaction for 5 h, the pH was neutralized to 5 with hydrochloric acid, and the precipitated solid was mixed with ethanol and water. Recrystallization in solution (water content is 50%wt) obtains pure product 3-hydroxy-2-(4-iodophenyl)-4 hydrogen-chromen-4-one;
[0069] Add 8 mmoles of 3-hydroxyl-2-(4-iodophenyl)-4 hydrogen-chromen-4-one and 24 mmoles of dimethyl sulfate into the reactor to protect 3-hydroxyl-2-(4- The hy...
Embodiment 2
[0072] Preparation of flavonoid-based fluorescent dye 2-(4-(cyclopropylamino)phenyl)-3-hydroxy-4hydro-chromen-4-one
[0073] Add 10 millimoles of o-hydroxyacetophenone and 10 millimoles of p-iodobenzaldehyde into the reactor, add 30 milliliters of ethanol to dissolve, then add 30 milliliters of sodium hydroxide aqueous solution (mass concentration is 20%), and react at 70 degrees Celsius for 12 After one hour, the reaction system was placed in ice-water mixture to cool, and 10 mL of hydrogen peroxide (mass concentration was 30%) was added. After continuing the reaction for 5 h, the pH was neutralized to 5 with hydrochloric acid, and the precipitated solid was mixed with ethanol and water. Recrystallization from solution (water content 50%wt) gave pure 3-hydroxy-2-(4-iodophenyl)-4hydro-chromen-4-one.
[0074] Add 8 mmoles of 3-hydroxyl-2-(4-iodophenyl)-4 hydrogen-chromen-4-one and 24 mmoles of dimethyl sulfate into the reactor to protect 3-hydroxyl-2-(4- The hydroxyl group on ...
Embodiment 3
[0077] Preparation of flavonoid fluorescent dye 2-(4-(cyclobutylamino)phenyl)-3-hydroxy-4hydro-chromen-4-one (APHC)
[0078] Add 10 millimoles of o-hydroxyacetophenone and 10 millimoles of p-iodobenzaldehyde into the reactor, add 30 milliliters of ethanol to dissolve, then add 30 milliliters of sodium hydroxide aqueous solution (mass concentration is 20%), and stir the reaction at 60 degrees Celsius After 12 hours, the reaction system was cooled in a mixed solution of ice and water, and 10 mL of hydrogen peroxide (mass concentration: 30%) was added. After continuing the reaction for 4 hours, the pH was neutralized to 5 with hydrochloric acid, and the precipitated solid was dissolved in ethanol and water. Recrystallization in the mixed solution (water content is 50%wt) to obtain pure product 3-hydroxyl-2-(4-iodophenyl)-4hydro-chromen-4-one;
[0079] Add 8 mmoles of 3-hydroxyl-2-(4-iodophenyl)-4 hydrogen-chromen-4-one and 24 mmoles of dimethyl sulfate into the reactor to protect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com