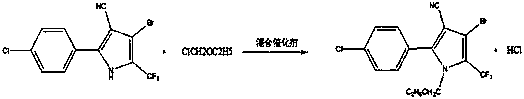
Production process for catalytic synthesis of chlorfenapyr with mixed catalyst
A technology for synthesizing chlorfenapyr and mixed catalysts, which is applied in the field of acaricide preparation, mixed catalysts catalyzing the synthesis of chlorfenapyr, and arylpyrrole compounds. It can solve the problem of prolonged reaction time, waste of water resources, and increased production costs. and other issues to achieve the effect of simplifying the production process, reducing product costs and saving labor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of mixed catalyst catalyzes the production process of synthesizing chlorfenapyr, the concrete operation is as follows:
[0036] (1) Feeding
[0037] Open the reactor manhole, 4-bromine 1050kg, mixed catalyst 252kg (93.6kg of potassium carbonate
[0038] and solid alkali 158.4kg) into the reactor, squeeze into the metered acetonitrile 3150kg, start stirring, and heat up.
[0039] (2) Add chlorine ether dropwise
[0040] When the temperature rises to 53~54°C, open the bottom valve of the chlorine ether high tank, and drop in 352kg of the measured chlorine ether.
[0041] (3) Insulation reaction
[0042] After the dropwise addition was completed and kept warm for 40 minutes, a sample was taken to analyze 4-bromine 0.11%, and the reaction was qualified.
[0043] (4) Solid-liquid separation
[0044] Solid-liquid separation was carried out by a solid-liquid separator, the solid was washed with 100 kg of acetonitrile, and 330 kg of filter cake was cleared out, and ...
Embodiment 2
[0049] A kind of mixed catalyst catalyzes the production process of synthesizing chlorfenapyr, the concrete operation is as follows:
[0050] (1) Feeding
[0051] Open the reactor manhole, 4-bromine 1050kg, mixed catalyst 252kg (93.6kg of potassium carbonate
[0052] and solid alkali 158.4kg) into the reactor, squeeze into the metered acetonitrile 3150kg, start stirring, and heat up.
[0053](2) Add chlorine ether dropwise
[0054] When the temperature rises to 54~55°C, open the bottom valve of the chlorine ether high tank, and drop in 352kg of the measured chlorine ether.
[0055] (3) Insulation reaction
[0056] After the dropwise addition, keep warm for 40 minutes, take a sample and analyze 4-bromine 0.13%, and the reaction is qualified.
[0057] (4) Solid-liquid separation
[0058] Solid-liquid separation was carried out by a solid-liquid separator, the solid was washed with 100 kg of acetonitrile, and 340 kg of filter cake was cleared out, and the filtrates were comb...
Embodiment 3
[0063] A kind of mixed catalyst catalyzes the production process of synthesizing chlorfenapyr, the concrete operation is as follows:
[0064] (1) Feeding
[0065] Open the reactor manhole, 4-bromine 1050kg, mixed catalyst 252kg (93.6kg of potassium carbonate
[0066] and solid alkali 158.4kg) into the reactor, squeeze into the metered acetonitrile 3150kg, start stirring, and heat up.
[0067] (2) Add chlorine ether dropwise
[0068] When the temperature rises to 50~52°C, open the bottom valve of the chlorine ether high tank, and drop in 352kg of the measured chlorine ether.
[0069] (3) Insulation reaction
[0070] After the dropwise addition, keep warm for 40 minutes, take a sample and analyze 4-bromine 0.15%, and the reaction is qualified.
[0071] (4) Solid-liquid separation
[0072] Solid-liquid separation was carried out by a solid-liquid separator, the solid was washed with 100 kg of acetonitrile, and 335 kg of filter cake was cleared out, and the filtrates were com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com