Blood circulation miRNA biomarker assay kit for diagnosis and prognosis evaluation of gastric cancer
A technology of biomarkers and detection kits, applied in the field of kits, can solve the problems of insufficient protein stability, improper storage, easy degradation, and insurmountability, and achieve the effect of excellent diagnostic efficiency and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
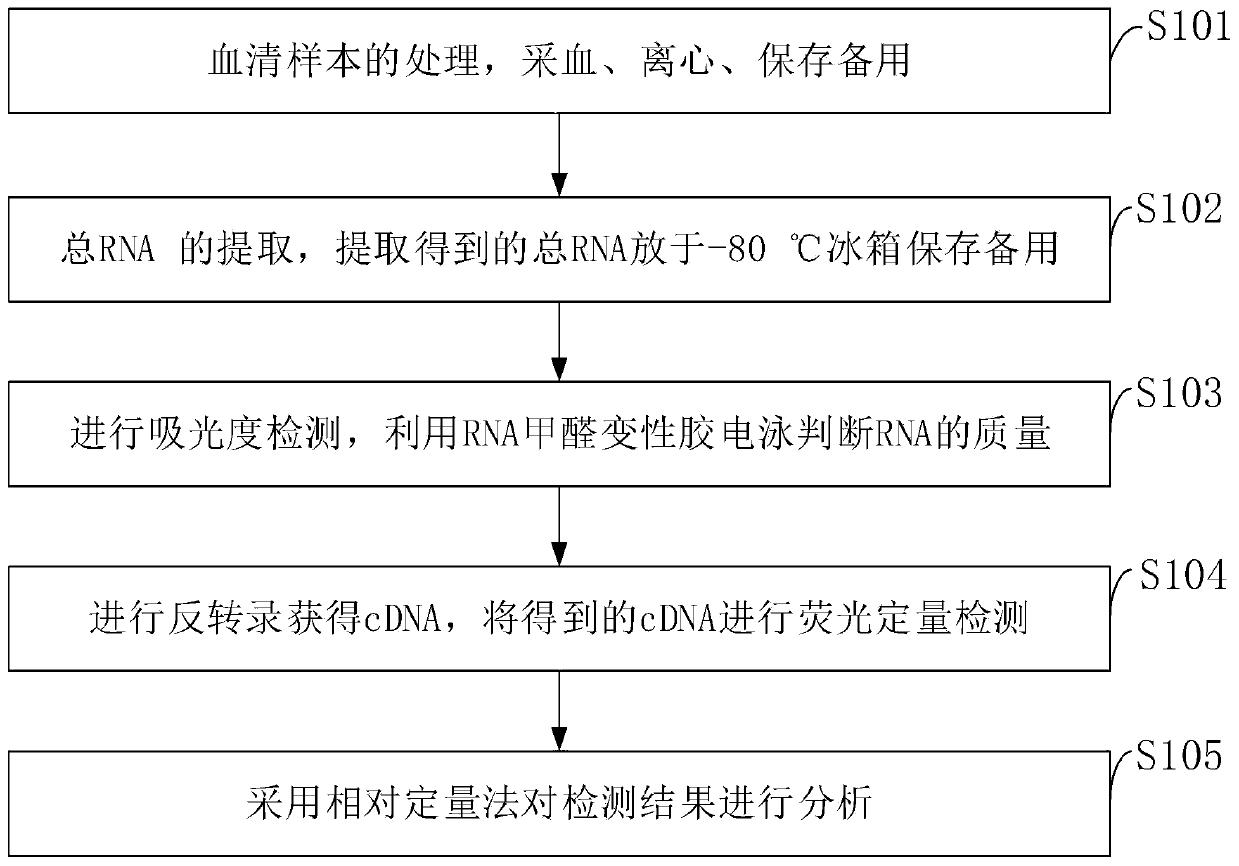
[0095] (1) Preparation of 2.5% agarose gel plate
[0096] Add 2.5g of agarose powder to 0.5×TBE100ml and heat it in a microwave oven until the powdered agarose is completely dissolved; when cooled to 60°C-70°C, add 10mg / ml ethidium bromide 5ul, pour into the sealed gel tank , with a thickness of about 3-5mm, insert the toothed comb; after cooling and forming, carefully pull out the toothed comb, put it into the agarose horizontal electrophoresis tank, and submerge the gel surface with 0.5xTBE until 1cm;
[0097] (2) Take 0.3 μg of total RNA, add 1 / 5 volume of 5× loading buffer, heat at 65°C for 5 minutes, and quench on ice to eliminate the secondary structure of RNA; add 0.5 μg to the RNA sample before loading -1.0μl ethidium bromide EtBr, concentration 1.0mg / mL; prepared 1.2% formaldehyde denaturing gel in 1×formaldehyde denaturing gel electrophoresis buffer for pre-electrophoresis for 15min; RNA samples at a voltage of 5-10V / cm Lower the electrophoresis for 30min.
[0098]...
Embodiment 1
[0104] Gastric cancer detection kit
[0105] (1) Nucleotide sequence mir-30c, specific miR-30c nucleotide sequence:
[0106] 5'-TGTAAACATCCTACACTCTCAGC-3'.
[0107] (2) U6 snRNA is used as an internal reference: the sequences of primers upstream and downstream of U6 primers are:
[0108] 5'-CTCGCTTCGGCAGCACA-3',
[0109] 5'-AACGCTTCACGAATTTGCGT-3'.
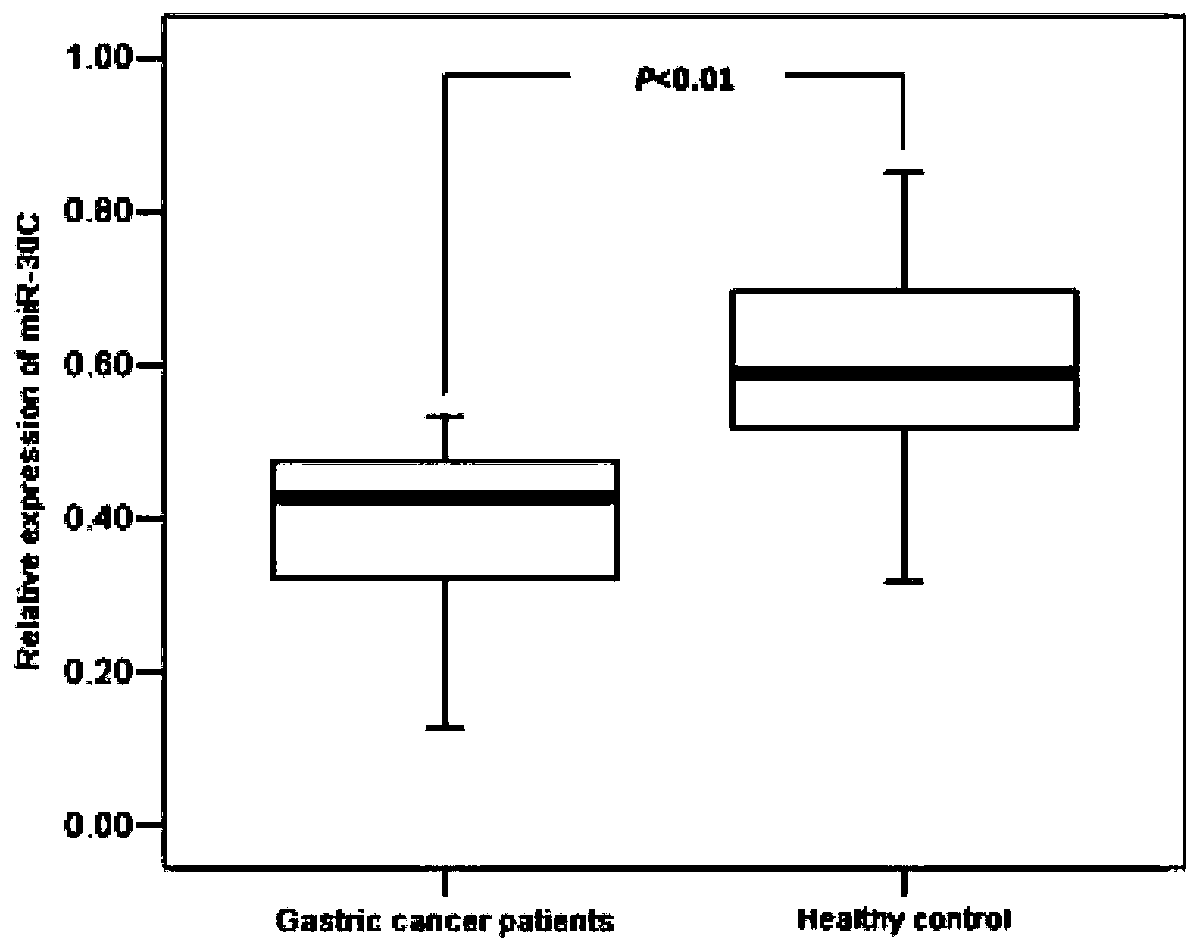
[0110] (3) Calculation method of relative expression of miR-30c: 2 -ΔCt Cycle value calculation, ΔCt=Ct miR-30c -Ct U6
[0111] 1. Instrument
[0112] The main instruments are as follows
[0113]
[0114]
[0115] 2. Kit detection steps
[0116] 1. Kit detection:
[0117] 1.1 Treatment of serum samples All the included populations took 3ml of fasting venous blood in the morning, and immediately centrifuged with a horizontal centrifuge at 2000rpm for 8min to separate the serum. The supernatant was placed in a new centrifuge tube and stored in a -80°C refrigerator for extraction of total RNA. Extraction of total RN...
Embodiment 2
[0163] 2.1 Sample collection:
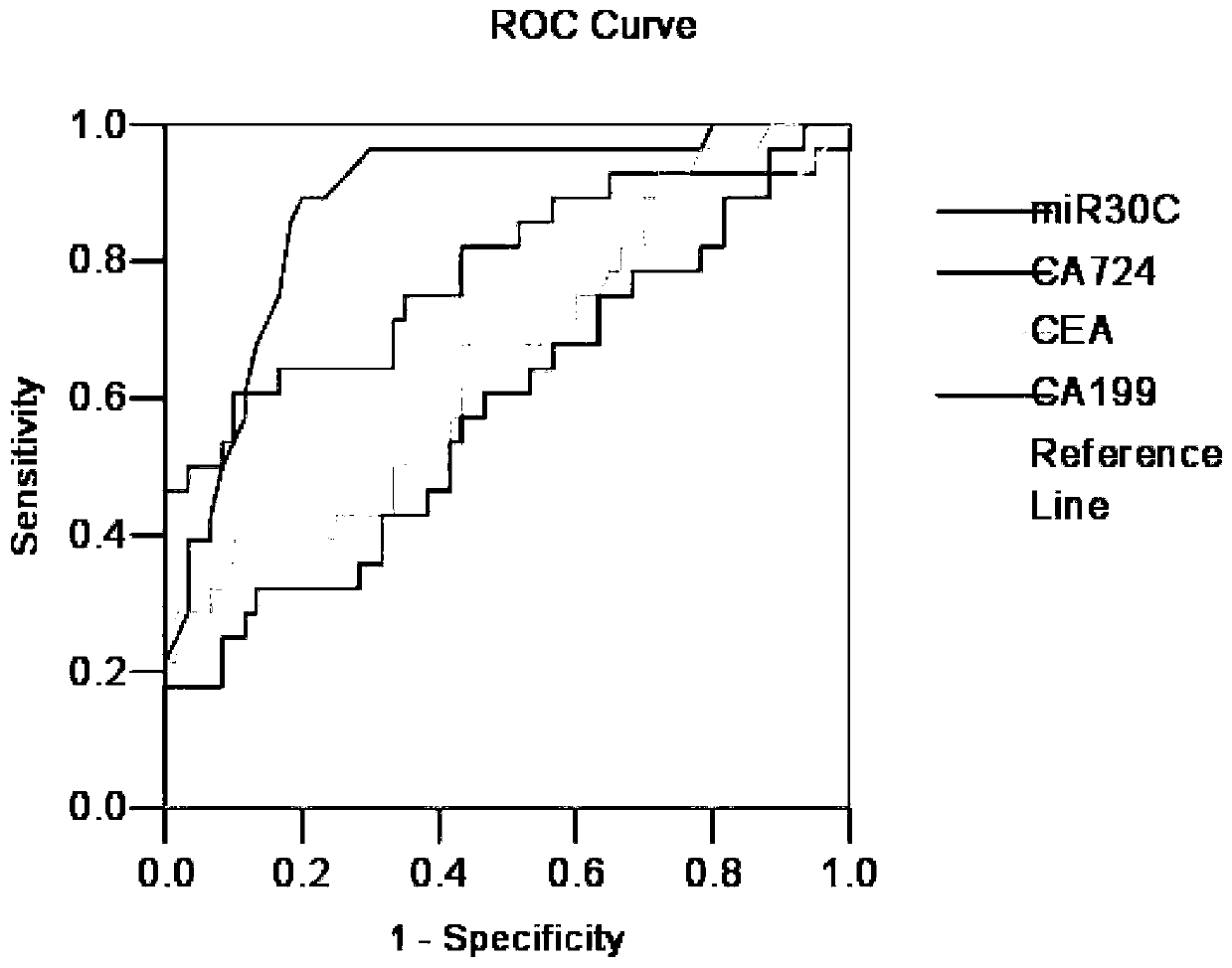
[0164] Conduct clinical sample research in Inner Mongolia Autonomous Region, China. A total of 240 inpatient gastric cancer surgery samples were collected from the First Affiliated Hospital of Inner Mongolia Medical College from January 2006 to October 2011. The removed gastric cancer tissue samples were immediately frozen in liquid nitrogen and then stored at -80°C in the refrigerator. The above surgical resection samples were independently diagnosed as gastric cancer by two senior pathologists, and pathological data were collected after pathological examination. The depth of tumor invasion is classified according to UICC (International Union Against Cancer standard) [19] , the status of lymph node metastasis, and the degree of differentiation were judged according to WHO (World Health Organization) standards [20] , The location of the tumor is subject to the pathological report.
[0165] Non-cancerous samples matched by sex, age and gastri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com