Nickle-based catalyst and preparation method and application thereof
A catalyst and nickel-based technology, applied in the field of nickel-based catalysts and their preparation, can solve the problems of high catalyst cost, low pentane yield, catalyst deactivation and the like, and achieve good hydrogenation activity, simple preparation method and high stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The method for preparing the catalyst as described in the first aspect of the present invention related to the second aspect of the present invention, it comprises the following steps:
[0027] S1, after impregnating the alumina support with a solution containing rare earth metals, drying and roasting to obtain a modified alumina support modified with rare earth metals;
[0028] S2, after impregnating the rare earth metal-modified alumina carrier in a nickel salt solution, drying and calcining to obtain a nickel-based catalyst.
[0029] In some embodiments of the present invention, in steps S1 and S2, the calcination temperature is 300-800°C; the calcination time is 3-8h.
[0030] In other embodiments of the present invention, in step S2, the pH value of the nickel salt solution is 5-6; the soaking time is 3-5h.
[0031] In some specific embodiments of the present invention, the method for preparing the catalyst as described in the first aspect of the present invention...
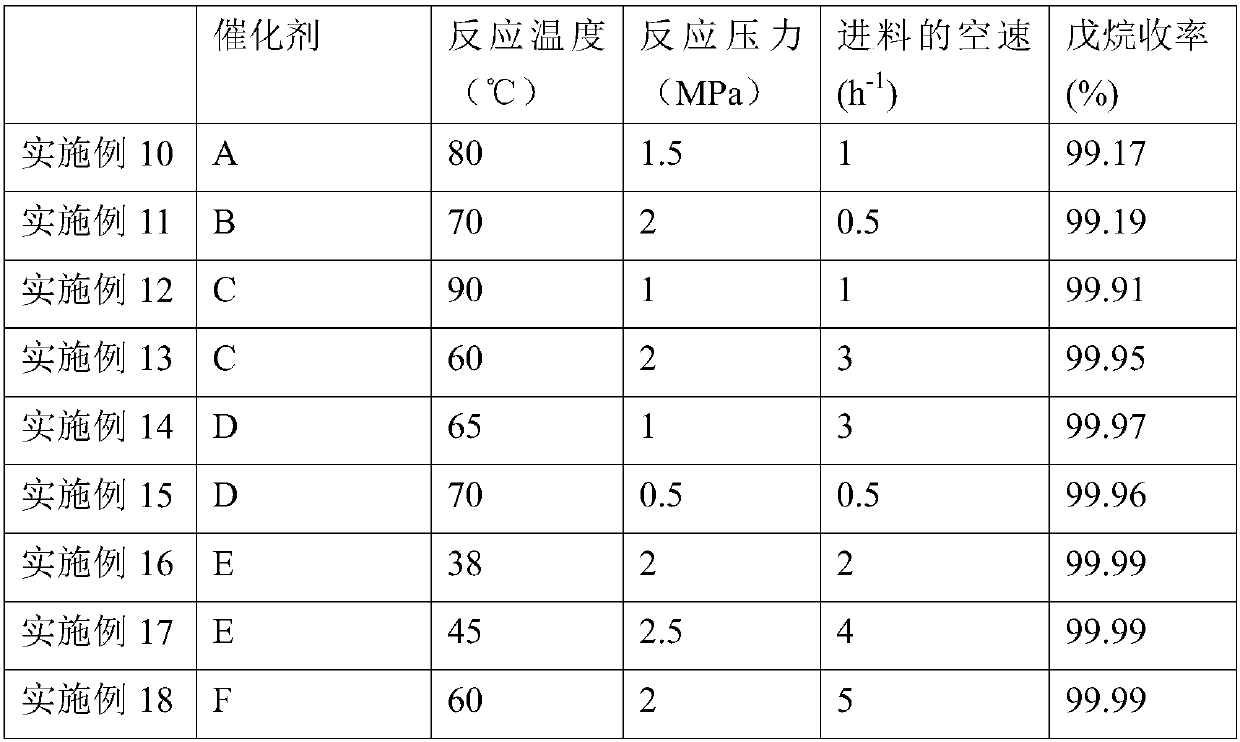
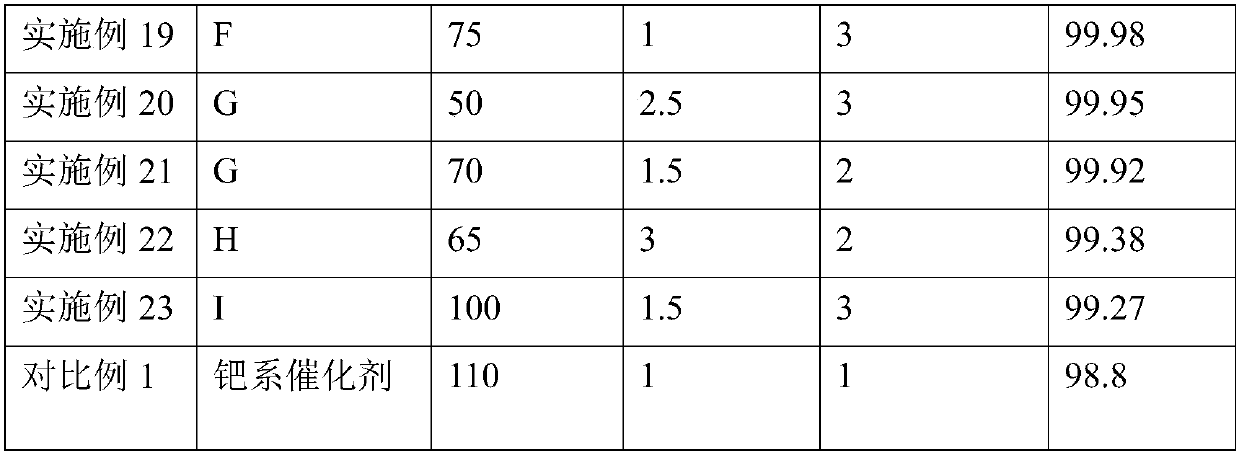
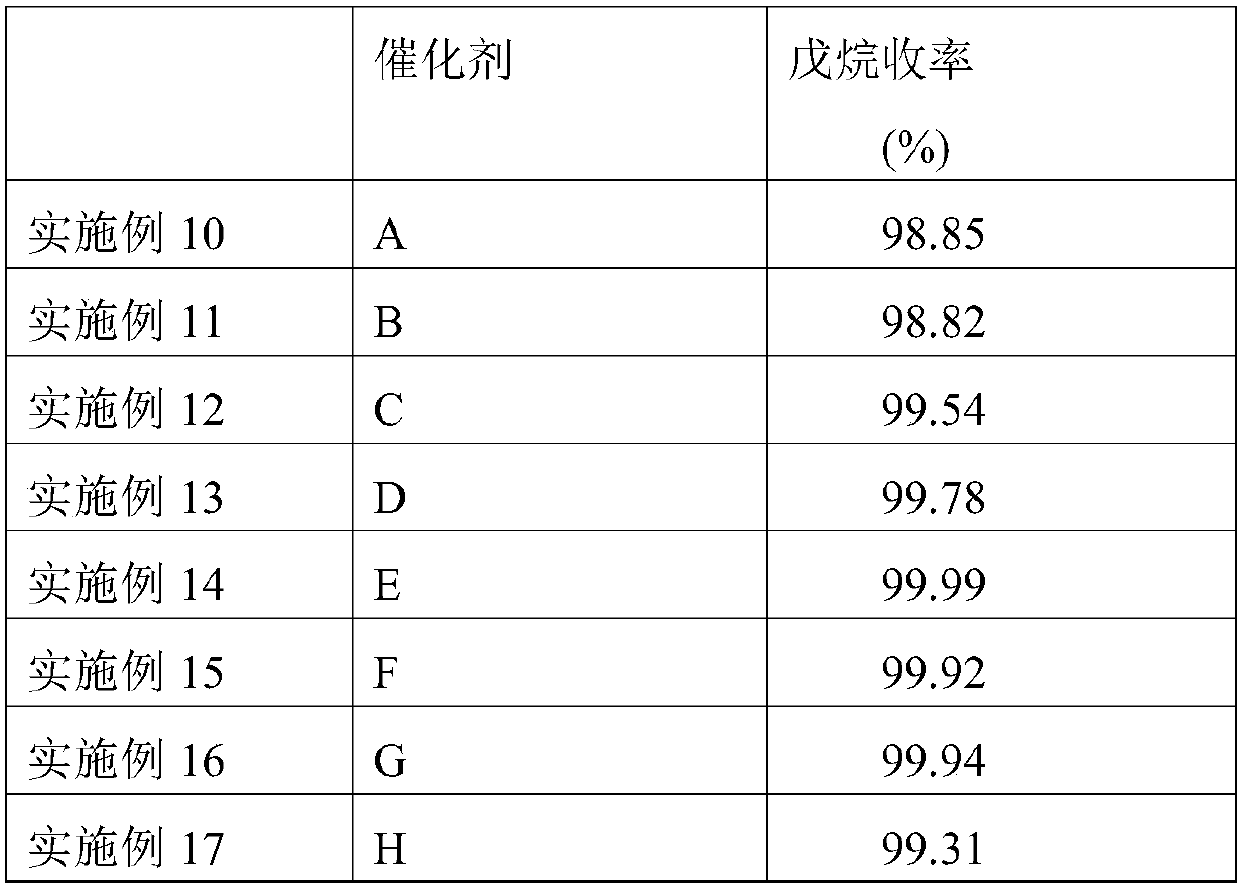
Embodiment 1
[0045] Embodiment 1: the preparation of nickel-based catalyst
[0046] (1) Preparation of modified alumina carrier
[0047]Make a solution of appropriate amount of lanthanum oxide and cerium oxide and add it to aluminum glue, heat and stir thoroughly, then filter and extrude, dry at 120°C for 10 hours, and bake at 600°C for 4 hours to obtain A modified alumina support containing 2.0 wt% lanthanum oxide and 1.0 wt% ceria.
[0048] (2) Preparation of nickel-based catalyst
[0049] Add 58.2g of nickel nitrate into 200mL of water, stir and dissolve, adjust its pH value to 5, and form a nickel nitrate solution. 100 g of the modified alumina support was immersed in the above nickel nitrate solution, dried at 120° C. for 6 hours, and then calcined at 600° C. for 5 hours to obtain nickel-based catalyst A. Wherein the content of nickel oxide is 15wt%.
Embodiment 2-9
[0051] Using the same method as in Example 1, nickel-based catalysts B, C, D, E, F, G, H, and I were obtained by changing the content of the active component nickel oxide. The composition of each catalyst is shown in Table 1.
[0052] Table 1: Composition of the nickel-based catalysts obtained in Examples 2-9
[0053] Catalyst number Nickel oxide content wt% Example 2 B 17 Example 3 C 18 Example 4 D 19 Example 5 E 20 Example 6 F 21 Example 7 G 22 Example 8 H 24 Example 9 I 25
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com