Hepatitis B virus (HBV) DNA quantitative detection kit
A technology of hepatitis B virus and kit, which is applied in the direction of recombinant DNA technology, DNA/RNA fragments, microbial measurement/inspection, etc. It can solve the problems that digital PCR cannot be directly applied, and achieves the reduction of false positive detection results and high detection efficiency. Yield, simple design effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: The kit that is used for quantitative determination HBV DNA in human serum or plasma
[0105] This kit is used for the quantitative determination of HBV DNA in human serum or plasma. The reagent components included in the kit are as follows:
[0106] kit components main ingredient dPCR Reaction Master Mix HBV specific primers, probes, hot start Taq enzyme, UDG enzyme, dNTPs and MgCl 2
External reference quality control Marked recombinant plasmids containing external reference gene fragments HBV Negative Quality Control Labeled inactivated HBV positive serum or plasma HBV Strong Positive Quality Control Labeled inactivated HBV positive serum or plasma HBV Weak Positive Quality Control Labeled inactivated HBV positive serum or plasma
[0107] First, sample processing and nucleic acid extraction are performed. The sample is serum or plasma; the sample volume is 0.5-2 mL, preferably 1 mL. The external refe...
Embodiment 2
[0126] Example 2: Sensitivity
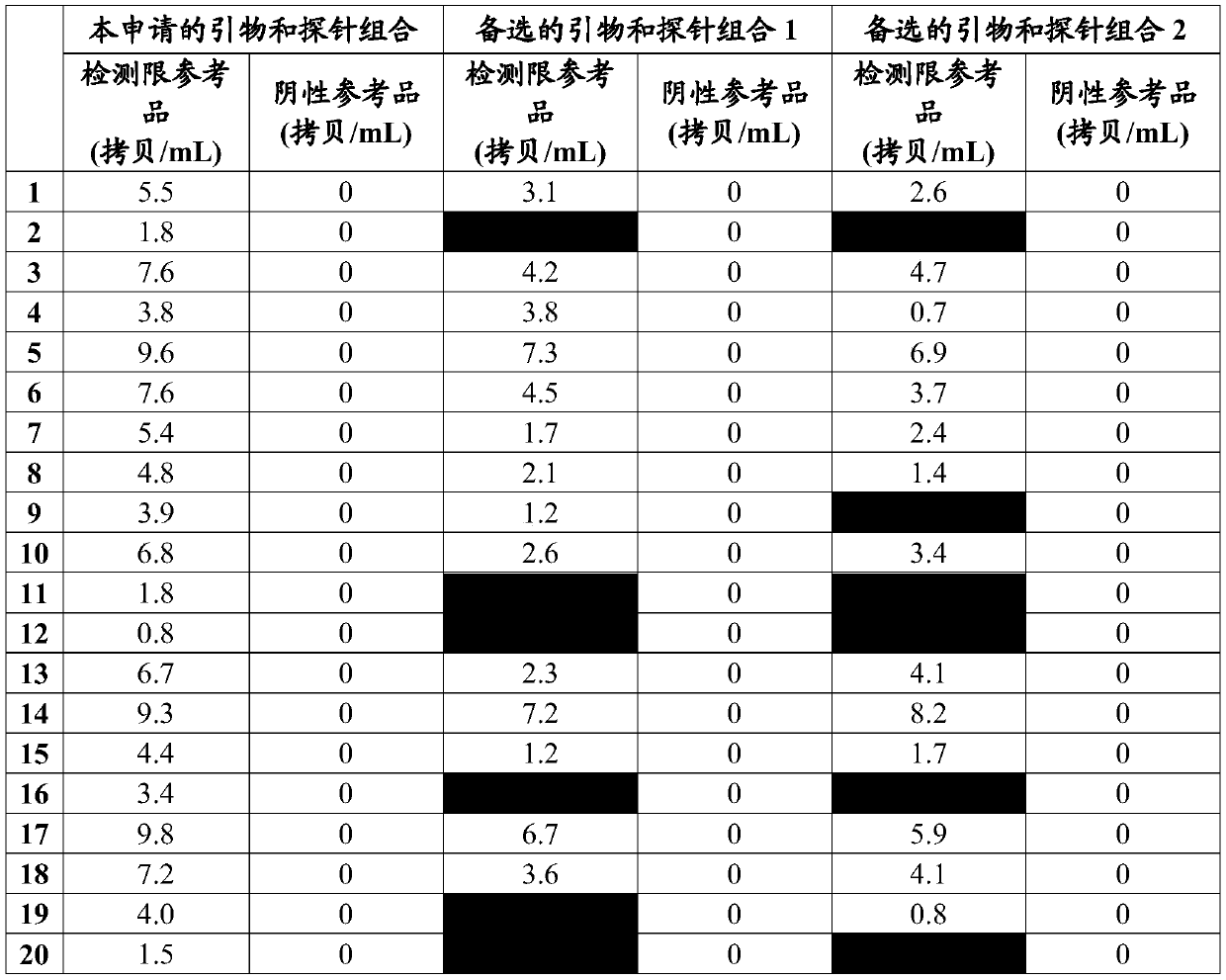
[0127] The detection limit reference product is the clinical HBV DNA sample determined by the national reference product, and the HBV negative serum is used to dilute the sensitivity of the kit to 10IU / mL as the detection limit reference product (20 repetitions). According to the steps in Example 1, the detection sensitivity involved in this application was evaluated. The actual measured results are shown in Table 1 below:
[0128]
[0129] The primers and probes for this application in the table above are as follows:
[0130] Forward primers include: primers shown in SEQ ID NO.:1 and SEQ ID NO.:2,
[0131] Reverse primers include: primers shown in SEQ ID NO.:3,
[0132] Probes include: the probes shown in SEQ ID NO.:4 and SEQ ID NO.:5.
[0133] Alternative primers and probes are different from the primers and probes of the present application for the same site design of hepatitis B virus (HBV) DNA, and its specific sequence is as follows...
Embodiment 3
[0143] Example 3: Accuracy
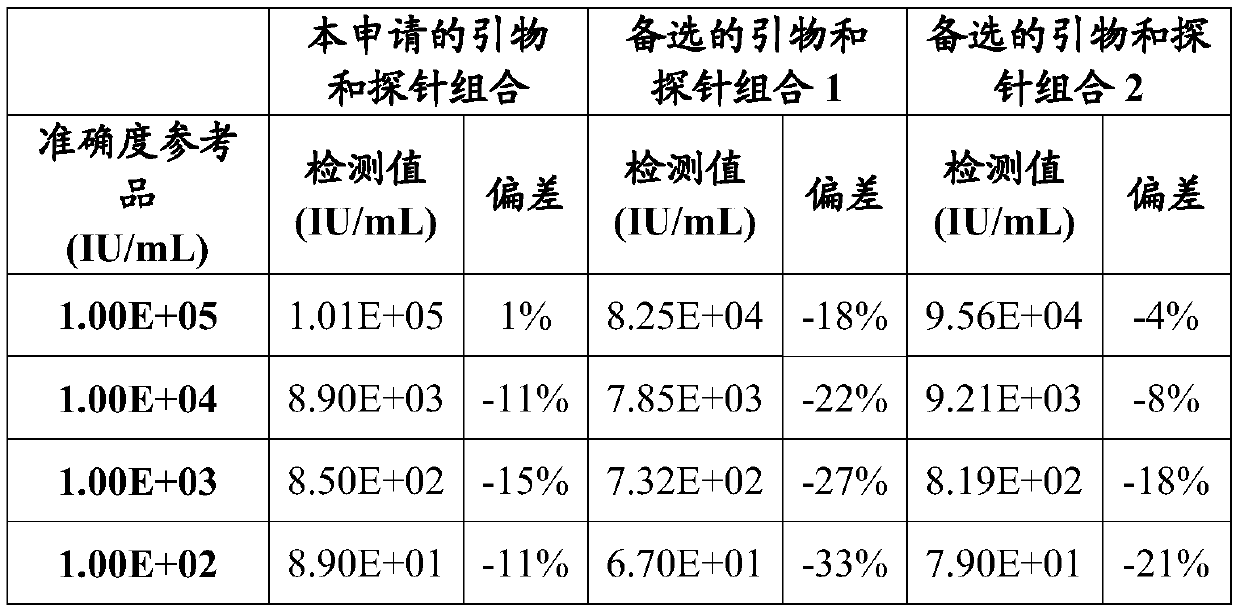
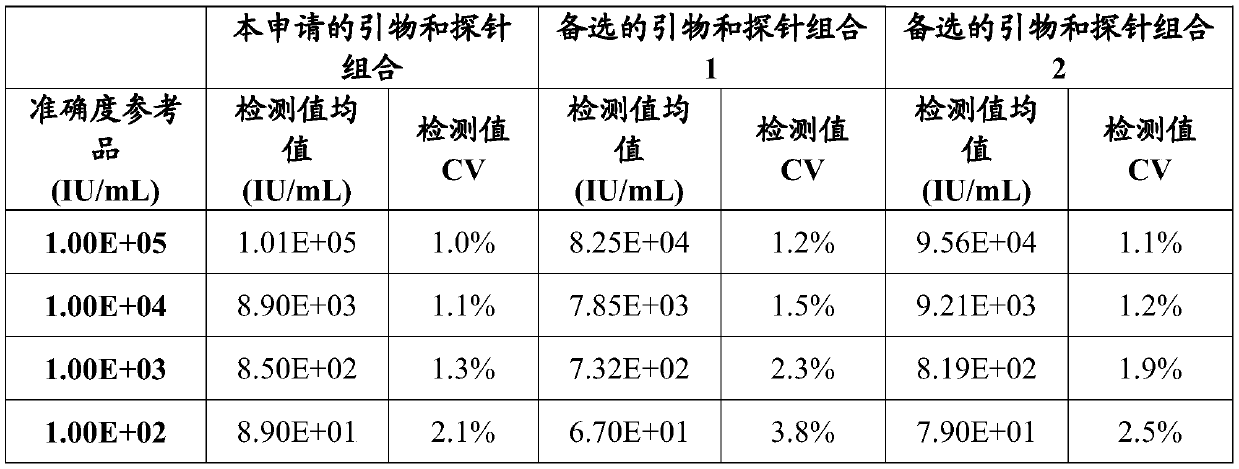
[0144] The accuracy reference product is the clinical HBV DNA sample determined by the national reference product, which is diluted with HBV negative plasma to 1.0×10 5 IU / ml, 1.0×10 4 IU / ml, 1.0×10 3 IU / ml and 1.0×10 2 IU / ml, as a reference product for accuracy, a total of 4. Nucleic acid extraction was carried out with the accuracy reference product, HBV DNA negative quality control, HBV DNA strong positive quality control and HBV weak positive quality control. According to the steps in Example 1, the detection accuracy involved in this application is evaluated. The actual measured results are shown in Table 2 below:
[0145]
[0146] The test results show that: in the test of 4 accuracy reference products, in 10 2 -10 5 Within the detection range of IU / mL, the deviations detected by the primers and methods involved in the present application are all less than 20%, and the accuracy is high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com