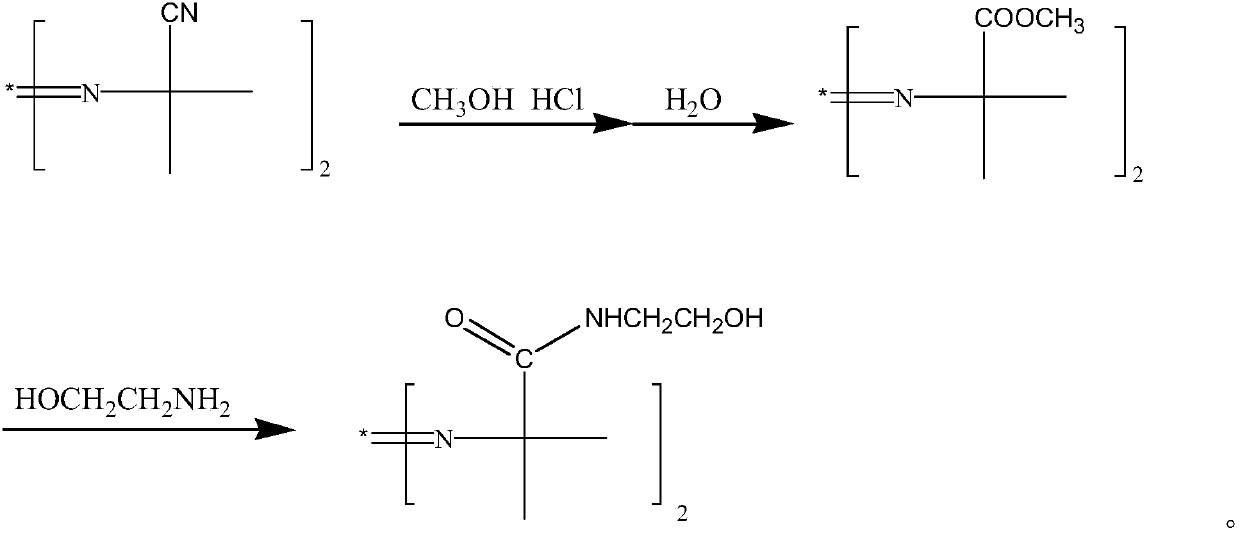
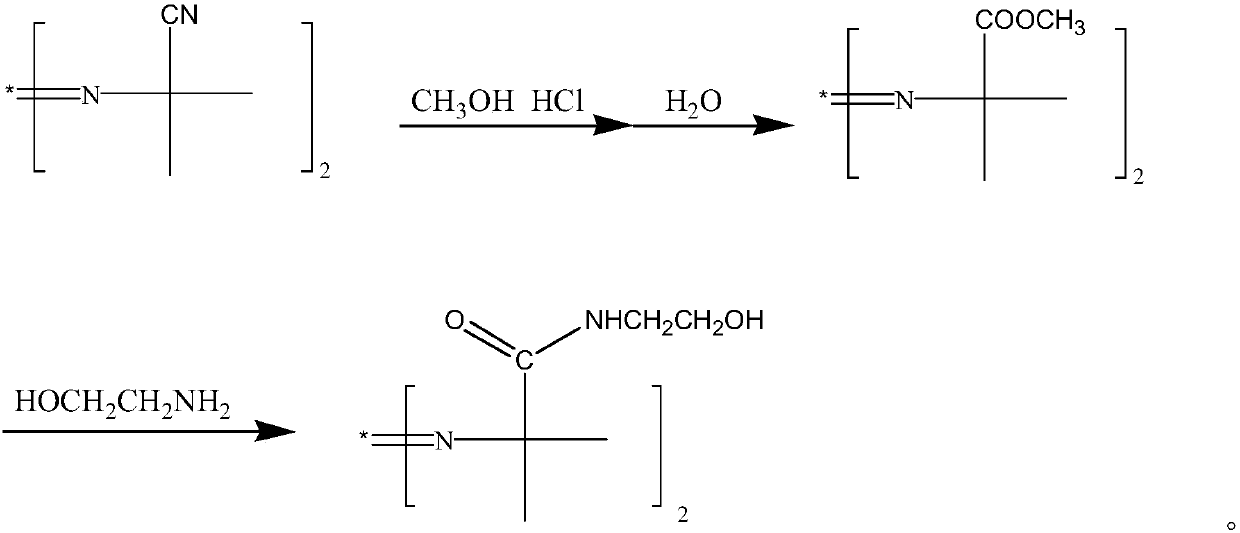
2,2'-azo(2-methyl-N-(2-hydroxyethyl)propionamide) preparation method
A technology of hydroxyethyl and propionamide, applied in the direction of organic chemistry, can solve the problems of material loss, difficult layering operation, serious environmental pollution, etc., and achieve the effect of easy operation, clear layering interface, and avoiding material loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 500 ml three-necked flask with a stirring and reflux device, first drop 41.8 grams of 98% 2,2'-azobisisobutyronitrile (0.25mol) and 200 grams of toluene, and then drop into 18.4 grams of 99.9% methanol (0.575mol) , start stirring, and start to slowly feed 19.4 grams of 99% hydrogen chloride gas (0.525 mol) under cooling at 15° C., wherein the rate of hydrogen chloride gas feeding is 38 g / hour.
[0034] Then control the material temperature at 18°C and keep it warm for 38 hours. After the reaction, the reaction solution was dripped into water at a rate of 110 g / h for hydrolysis for 0.5 hours. After the hydrolysis, the layers were separated. 12 grams of methanol was added to the organic phase, and the vacuum distillation was carried out for 2 hours. The pressure of the vacuum distillation was -0.097 MPa.
[0035] After the distillation, the organic phase was dripped into 31.5 grams of 30% sodium methylate solution (sodium methylate 0.175mol) and 33.5 grams of 98% m...
Embodiment 2
[0038] In a 500 ml three-necked flask with a stirring and reflux device, first drop 41.8 grams of 98% 2,2'-azobisisobutyronitrile (0.25mol) and 200 grams of toluene, and then drop into 16.4 grams of 99.9% methanol (0.5125mol) , start stirring, and start to feed 20.3 grams of 99% hydrogen chloride gas (0.55 mol) slowly under cooling at 15° C., wherein the hydrogen chloride gas feeding rate is 45 g / hour.
[0039] Then control material temperature 22 ℃, keep warm for 38 hours. After the reaction, the reaction solution was dripped into water at a rate of 110 g / hour to carry out the hydrolysis reaction for 1.5 hours. After the hydrolysis, the layers were separated, and 18 grams of methanol was added to the organic phase, and the vacuum distillation was carried out for 2.5 hours. The pressure of the vacuum distillation was -0.099MPa .
[0040] After the distillation, the organic phase was dripped into 27.0 grams of 30% sodium methylate solution (sodium methylate 0.15mol) and 32.7 g...
Embodiment 3
[0043] In a 500 ml three-necked flask with a stirring and reflux device, first drop 41.8 grams of 98% 2,2'-azobisisobutyronitrile (0.25mol) and 200 grams of toluene, and then drop into 17.6 grams of 99.9% methanol (0.55mol) , start stirring, and start to feed 19.4 grams of 99% hydrogen chloride gas (0.525 mol) slowly under cooling at 15° C., wherein the hydrogen chloride gas feeding rate is 42 g / hour.
[0044] Then control material temperature 20 ℃, keep warm for 38 hours. After the reaction, the reaction solution was dripped into water at a rate of 110 g / h for hydrolysis for 1.5 hours. After the hydrolysis, the layers were separated. 20 g of methanol was added to the organic phase, and the vacuum distillation was carried out for 2.5 hours. The pressure of the vacuum distillation was -0.01 MPa.
[0045]After the distillation, the organic phase was dripped into 15.3 grams of 30% sodium methoxide solution (sodium methoxide 0.085mol) and 32.7 grams of 98% monoethanolamine (0.525m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com