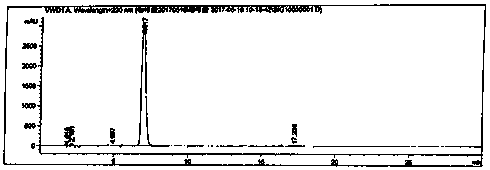
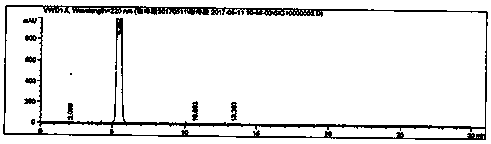
Caffeic acid synthesis and refining process
A caffeic acid and process technology, applied in the field of caffeic acid synthesis and refining process, can solve the problems of large amount of solvent, high production cost, and low product yield, and achieve the effects of avoiding organic solvent extraction, low cost, and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 prepares caffeic acid crude product
[0024] Take 30g of 3,4-dihydroxybenzaldehyde and 60.0g of malonic acid, add 300ml of N,N-dimethylformamide to dissolve, add 45mL of pyridine, and then raise the temperature to 70°C. Add 9 mL of aniline and react for 1 hour. After the reaction, the temperature of the reaction liquid was lowered to below 30°C, 1000ml of 2moL / L hydrochloric acid was added and stirred, and stirred at room temperature for 2h to crystallize. Suction filtration, the filter cake was washed twice with 300ml purified water. Blast drying at 50°C yielded 36.50 g of crude yellow caffeic acid, with a yield of 93.60%, a purity of 99.52%, and a content of 99.2%.
Embodiment 2
[0025] Embodiment 2 prepares caffeic acid crude product
[0026] Take 30g of 3,4-dihydroxybenzaldehyde, 60.0g of malonic acid and add 300ml of tetrahydrofuran to dissolve, add 45mL of pyridine, and then raise the temperature to 70°C. Add 9 mL of aniline and react for 1 hour. After the reaction, the temperature of the reaction liquid was lowered to below 30°C, 1000ml of 2moL / L hydrochloric acid was added and stirred, and stirred at room temperature for 2h to crystallize. Suction filtration, the filter cake was washed twice with 300ml purified water. Blast drying at 50°C yielded 36.38 g of crude yellow caffeic acid, with a yield of 93.28%, a purity of 99.61%, and a content of 99.0%.
Embodiment 3
[0027] Embodiment 3 prepares caffeic acid crude product
[0028] Take 30g of 3,4-dihydroxybenzaldehyde, 60.0g of malonic acid and add 300ml of methanol to dissolve, add 45mL of pyridine, and then raise the temperature to 70°C. Add 9 mL of aniline and react for 1 hour. After the reaction, the temperature of the reaction liquid was lowered to below 30°C, 1000ml of 2moL / L hydrochloric acid was added and stirred, and stirred at room temperature for 2h to crystallize. Suction filtration, the filter cake was washed twice with 300ml purified water. Blast drying at 50°C yielded 36.44 g of crude yellow caffeic acid, with a yield of 93.44%, a purity of 99.64%, and a content of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com