Crystallization method of iprodione
A technology of crystallization of iprodione, which is applied in the field of crystallization of iprodione, can solve problems such as low purity, high cost, and environmental protection, and achieve the effects of improving purity, improving crystal form, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
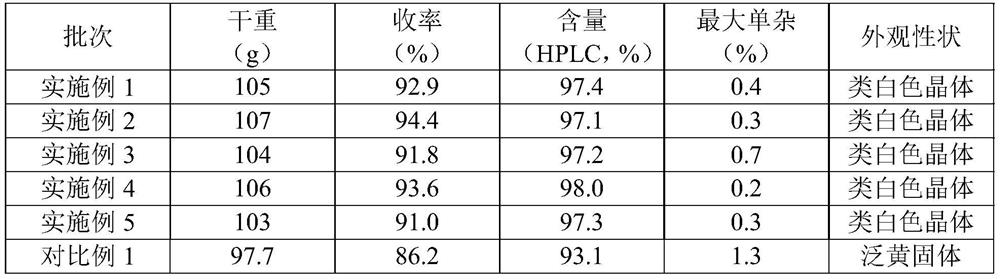
Embodiment 1
[0031] The present embodiment provides a crystallization method of iprodione, comprising the following steps:
[0032] Step (A), the preparation of iprodione
[0033] Feeding coefficient: toluene 400ml, hydantoin 85g, isopropyl isopropyl ester 40g, triethylamine 14g, catalyst 0.6g, condensation reaction occurs after mixing, pickling and water washing, and organic urea dissolved in iprodione is collected. Mutually.
[0034] Step (B), crystallization of iprodione
[0035] The toluene organic phase dissolved with iprodione was transferred to a 1000ml flask, and the organic solvent toluene was removed by a vacuum evaporator under negative pressure. When there is solid precipitation on the inner wall of the flask, about 280ml of toluene is removed at this time, and the acetic acid aqueous solution with pH=5 is started dropwise, and the toluene is removed under negative pressure while dropping. Control the dripping speed of acetic acid aqueous solution so that the dripping speed ...
Embodiment 2
[0038] The present embodiment provides a crystallization method of iprodione, comprising the following steps:
[0039] Step (A), the preparation of iprodione
[0040] Feeding coefficient: toluene 400ml, hydantoin 85g, isopropyl isopropyl ester 40g, triethylamine 14g, catalyst 0.6g, condensation reaction occurs after mixing, pickling and water washing, and organic urea dissolved in iprodione is collected. Mutually.
[0041] Step (B), crystallization of iprodione
[0042] The toluene organic phase dissolved with iprodione was transferred to a 1000ml flask, and the organic solvent toluene was removed by a vacuum evaporator under negative pressure. When there is solid precipitation on the inner wall of the flask, about 280ml of toluene is removed at this time, and the acetic acid aqueous solution with pH=6 is started dropwise, and the toluene is removed under negative pressure while dropping. Control the speed of acetic acid aqueous solution dripping, make the dropping speed of...
Embodiment 3
[0045] The present embodiment provides a crystallization method of iprodione, comprising the following steps:
[0046] Step (A), the preparation of iprodione
[0047] Feeding coefficient: toluene 400ml, hydantoin 85g, isopropyl isopropyl ester 40g, triethylamine 14g, catalyst 0.6g, condensation reaction occurs after mixing, pickling and water washing, and organic urea dissolved in iprodione is collected. Mutually.
[0048] Step (B), crystallization of iprodione
[0049] The toluene organic phase dissolved with iprodione was transferred to a 1000ml flask, and the organic solvent toluene was removed by a vacuum evaporator under negative pressure. When there is solid precipitation on the inner wall of the flask, remove about 280ml of toluene at this time, start to drop the acetic acid aqueous solution with pH=5.5, and continue to remove toluene under negative pressure while dropping, during this process, according to the speed of toluene removal To control the speed of the aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com