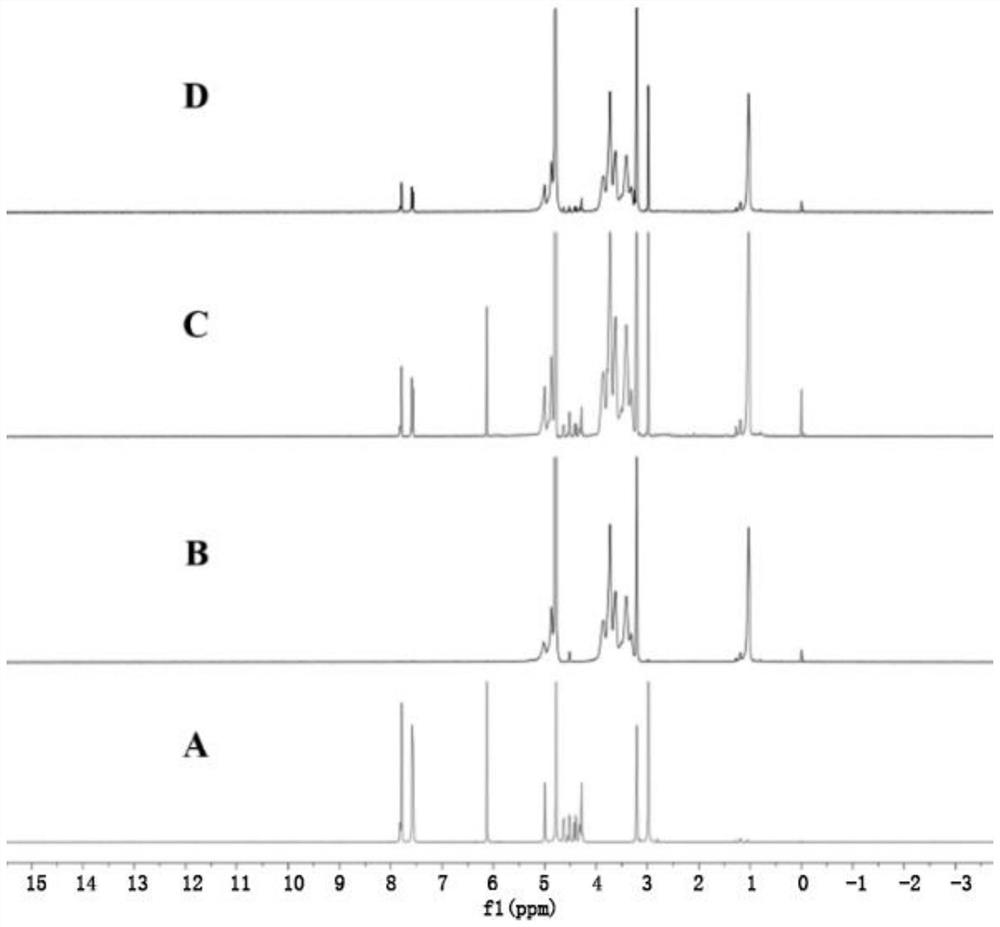
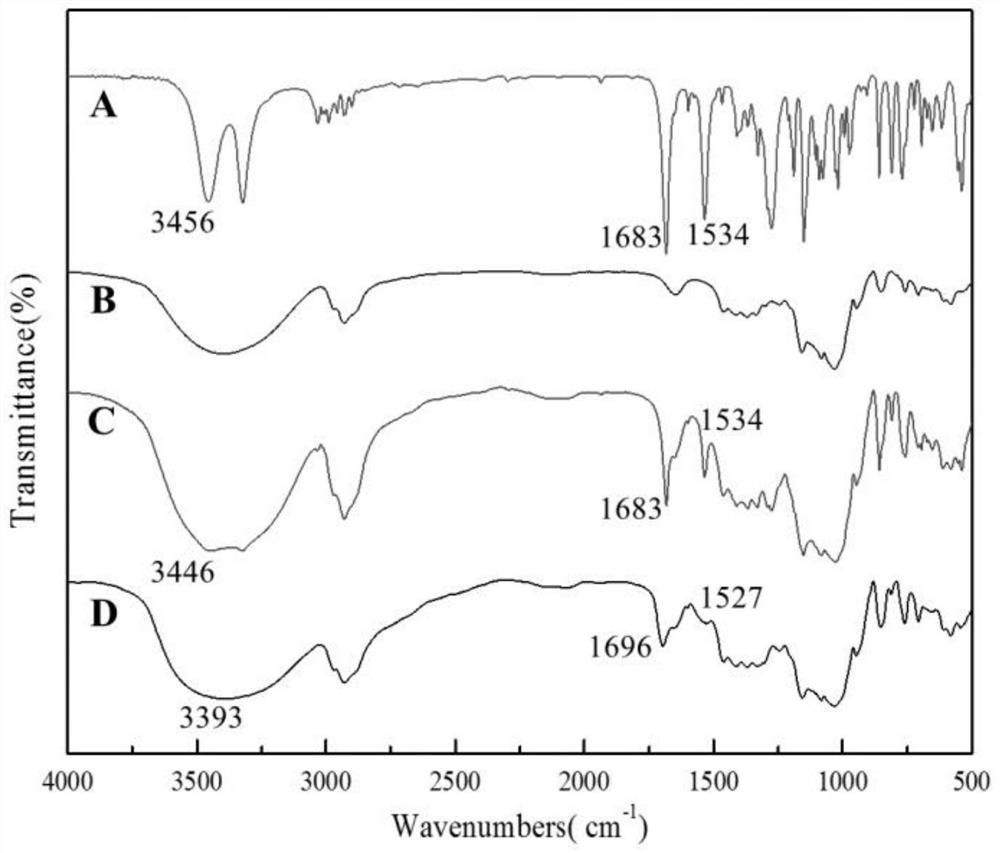
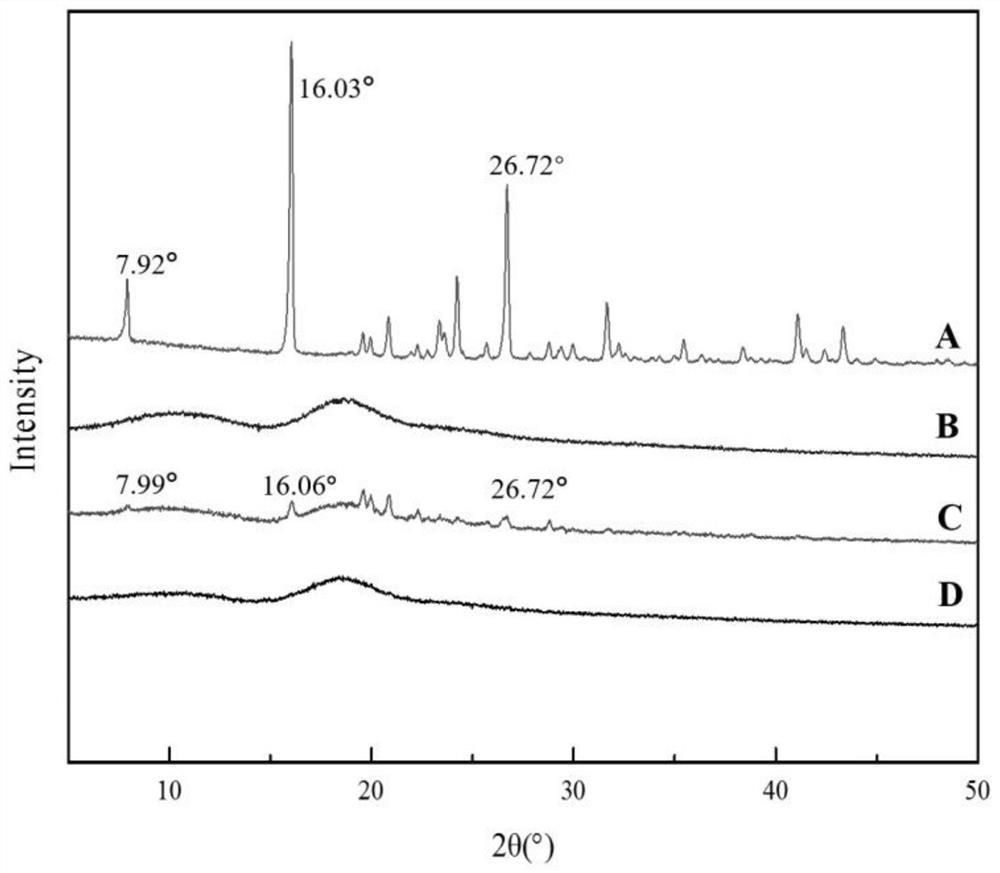
A kind of florfenicol inclusion compound freeze-dried powder injection and preparation method thereof
A technology of freeze-dried powder injection and florfenicol, which is applied in the field of florfenicol inclusion compound freeze-dried powder injection and its preparation, can solve the problems of small injection irritation, local injection irritation, poor solubility, etc., and achieve Effects of small injections on irritation, pain relief, and difficulty in administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A florfenicol inclusion compound freeze-dried powder injection, its preparation method is as follows:
[0041] (1) Weigh FF and HPCD, the molar ratio of HPCD and FF is 1:1;
[0042] (2) FF was dissolved in methanol with a concentration of 30%, HPCD was dissolved in an appropriate amount of water, and the mass concentration of HPCD in the aqueous solution was 10%, and the fully dissolved FF methanol solution was slowly added to the HPCD aqueous solution;
[0043] (3) Shake the obtained mixed solution in a constant temperature water bath shaker at 30° C. for 2 hours, the stirring speed is 100 r / min, and the pH of the reaction solution is 5.0;
[0044] (4) Evaporate the solvent completely on a rotary evaporator, and then filter the clathrate aqueous solution with a 0.22 μm microporous membrane;
[0045] (5) Dry in a vacuum freeze dryer, pre-freeze at -30°C for 4 hours, freeze-dry at -50°C for 16 hours, and dry at 30°C for 4 hours to obtain Florfenicol freeze-dried powder ...
Embodiment 2
[0048] A florfenicol clathrate lyophilized powder injection, the preparation process of which is basically the same as in Example 1, the difference is that in step (1), the molar ratio of HPCD to FF is 2:1; in step (2) , the mass concentration of HPCD in the aqueous solution is 15%; in step (3), the temperature is 50°C.
[0049] In Example 2, the drug loading of the clathrate aqueous solution prepared by step (4) was 10.07%, and the encapsulation efficiency was 86.95%; the freeze-dried powder injection obtained by step (5) had good appearance and resolubility, and the The powder is white, smooth and flat, and the solution is clear and transparent after reconstitution.
Embodiment 3
[0051] A florfenicol clathrate lyophilized powder injection, the preparation process of which is basically the same as in Example 1, the difference is that in step (1), the molar ratio of HPCD to FF is 2:1; in step (2) Among them, the mass concentration of HPCD in aqueous solution is 20%, and the concentration of methanol is 40%.
[0052] In Example 3, the drug loading of the clathrate aqueous solution prepared by step (4) was 10.12%, and the encapsulation efficiency was 87.40%; the freeze-dried powder injection obtained by step (5) had good appearance and resolubility, and the The powder is white, smooth and flat, and the solution is clear and transparent after reconstitution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com