Stereo-selective synthesis method of 6beta-hydroxymorphine derivative
A technology of stereoselectivity and synthesis method, which is applied in the preparation of sugar derivatives, sugar derivatives, and sugar derivatives, etc., can solve the problems of drug safety hazards, difficult removal of impurities, and low quality of M6G products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
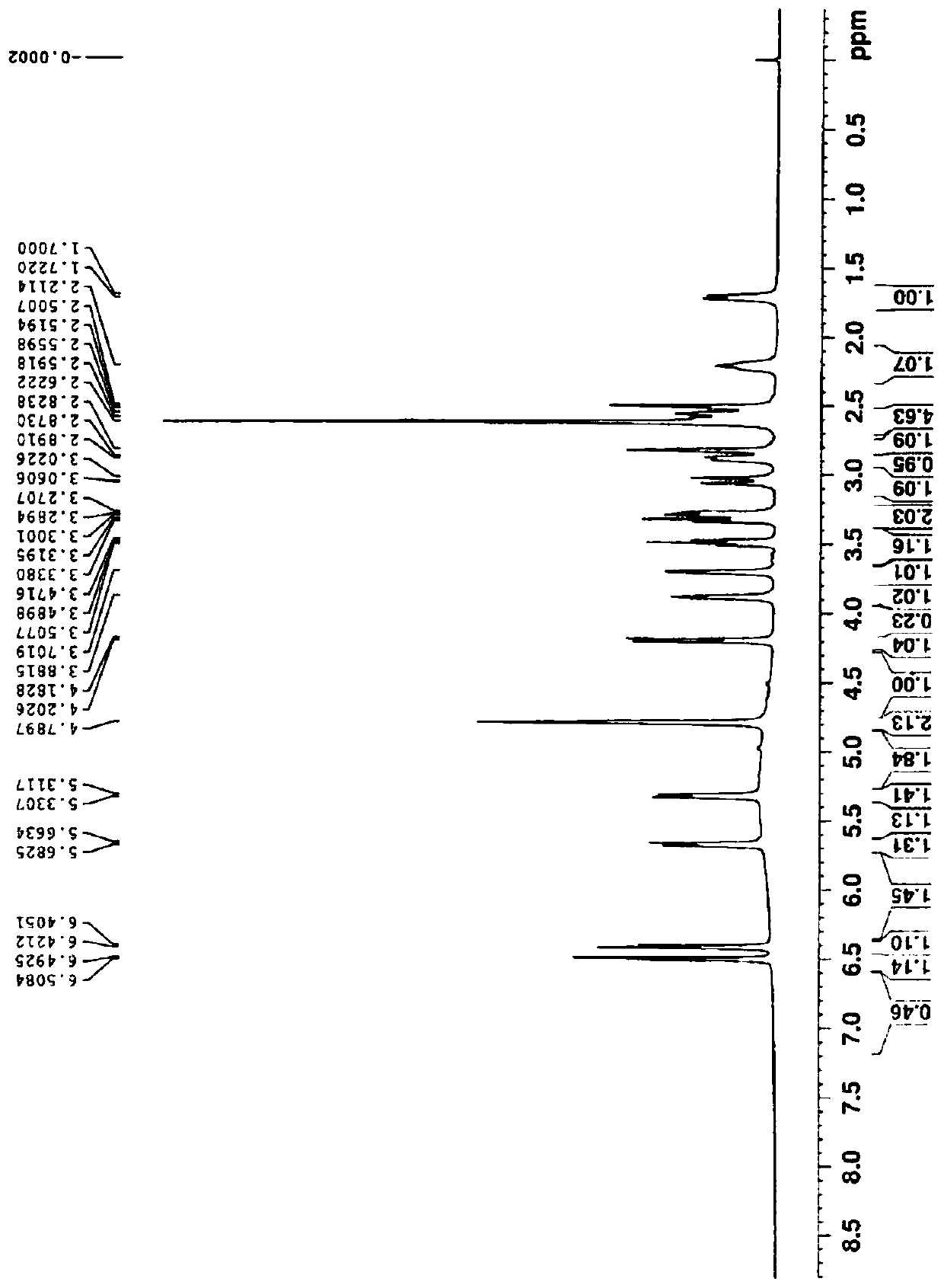
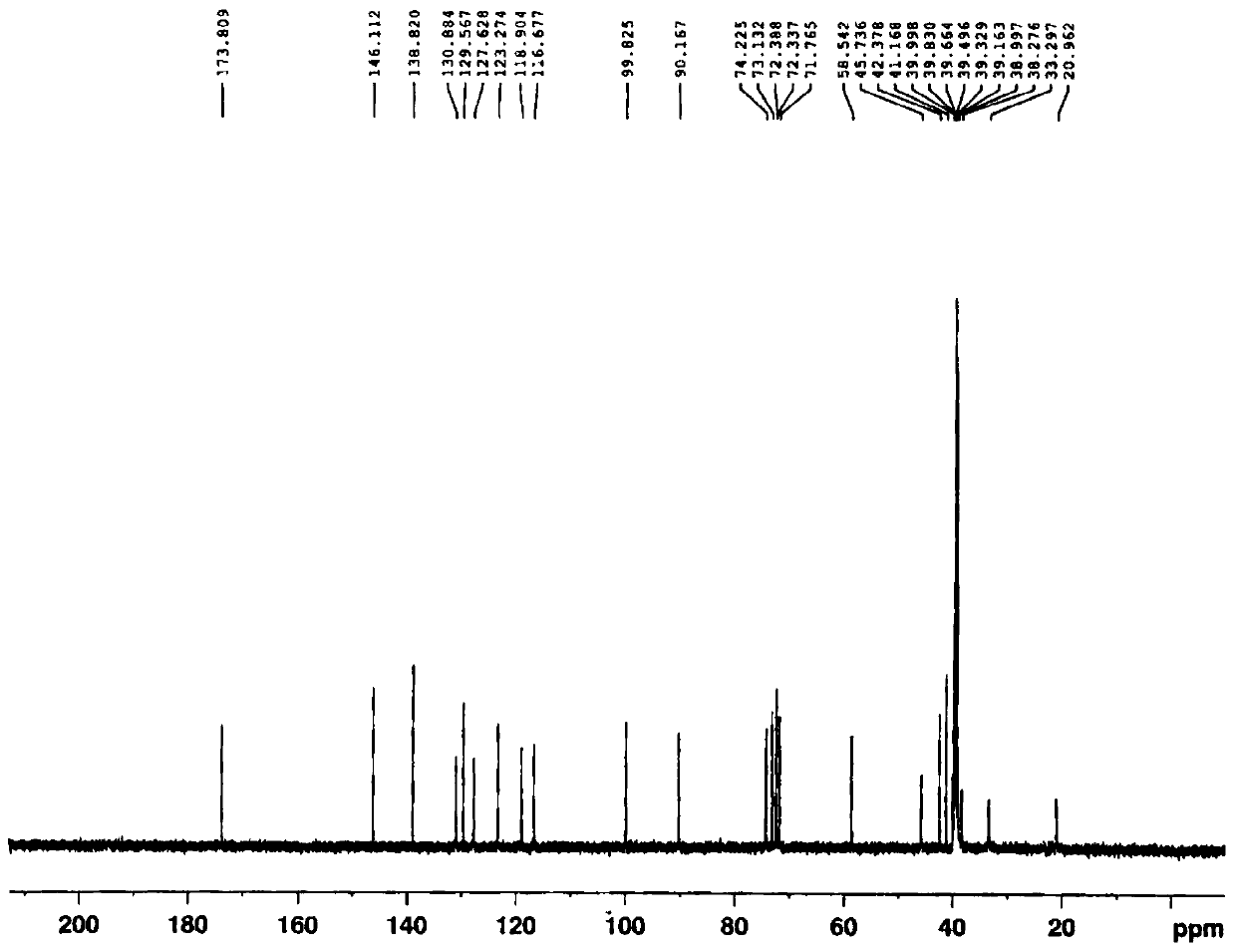
[0113] Morphine-6-β-O-β-D-glucuronide
[0114] S1: Add 33.4g (0.05mol) of morphine sulfate, 4g (0.1mol) of sodium hydroxide, 350mL of acetonitrile and 100ml of water into a 1L three-necked flask equipped with a thermometer, cool down to 10°C for reaction, and dropwise add 10.8g (0.05mol) 3,5-Dinitrobenzoyl chloride. After the dropwise addition, slowly return to room temperature and react for 5 hours. After the reaction is complete, pour the reaction solution into 500ml of ethyl acetate, separate the layers, extract the water layer twice with 200ml of ethyl acetate, combine the ethyl acetate layers, wash with 5% aqueous sodium bicarbonate until neutral, saturated chlorination Wash once with sodium, dry ethyl acetate overnight with anhydrous magnesium sulfate, filter, concentrate under reduced pressure at 45 ° C, and recrystallize the residue with ethanol to obtain 20.4 g of a white solid, namely the compound of formula II (R 1 is 3,5-dinitrobenzoyl, R 3 For methyl), yield: 8...
Embodiment 2
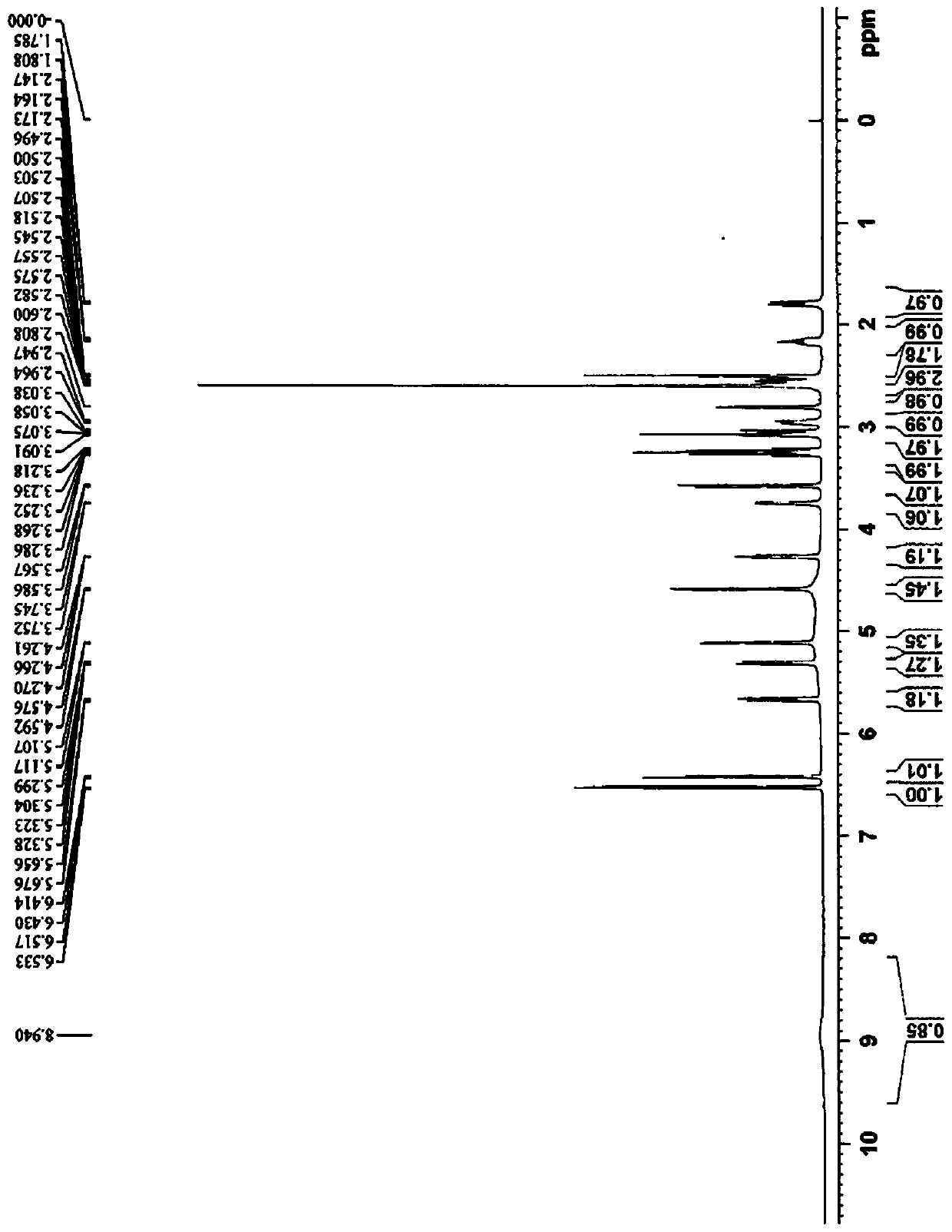
[0126] The preparation of embodiment 2 6-beta-hydroxymorphine
[0127]S1: Add 33.4g (0.05mol) of morphine sulfate, 4g (0.1mol) of sodium hydroxide, 350mL of acetonitrile and 100ml of water into a 1L three-necked flask equipped with a thermometer, cool down to 10°C for reaction, and add 5.3g (0.05mol) dropwise Isobutyryl chloride. After the dropwise addition, slowly return to room temperature and react for 5 hours. After the reaction is complete, pour the reaction solution into 500ml of ethyl acetate, separate the layers, extract the water layer twice with 200ml of ethyl acetate, combine the ethyl acetate layers, wash with 5% aqueous sodium bicarbonate until neutral, saturated chlorination Wash once with sodium, dry ethyl acetate overnight with anhydrous magnesium sulfate, filter, concentrate under reduced pressure at 45 ° C, and recrystallize the residue with ethanol to obtain 16.4 g of a white solid, namely the compound of formula II (R 1 is isobutyryl, R 3 For methyl), yi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com