Fc-binding protein having improved antibody separation ability, and method for separating antibody using same
A separation method and protein technology, which can be applied to the preparation methods of peptides, immunoglobulins, chemical instruments and methods, etc., can solve the problems of insufficient improvement of the separation degree and adsorption capacity of antibody analysis, and achieve the effect of improving the separation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1F
[0067] Preparation of Example 1 FcR9 Amino Acid Substitution Product
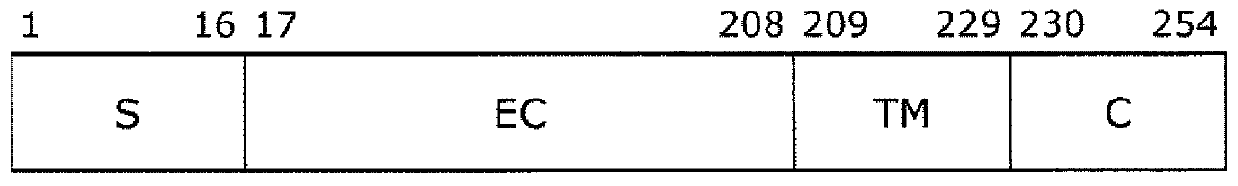
[0068] The amino acid substitution introduced into the Fc-binding protein FcR9 (SEQ ID NO: 5) was prepared according to the method disclosed in the following WO2015 / 199154 to confirm that the valine at position 192 (corresponding to that described in SEQ ID NO: 1) was replaced by a different amino acid. The usefulness of valine at position 176 in wild-type human FcγRIIIa composed of amino acid sequences). Specifically, amino acid substitutions were introduced by PCR into plasmid pET-FcR9 (WO2015 / 199154) containing a polynucleotide (SEQ ID NO: 6) encoding FcR9, thereby producing a 192nd FcR9 (SEQ ID NO: 5) having other amino acid substitutions. Valine-positioned Fc-binding protein. FcR9 (SEQ ID NO: 5) is an Fc-binding protein containing the wild-type FcγRIII extracellular domain described in SEQ ID NO: 4, wherein the Val amino acid at position 43 is replaced by Glu (corresponding to position 27 in SEQ ID ...
Embodiment 2F
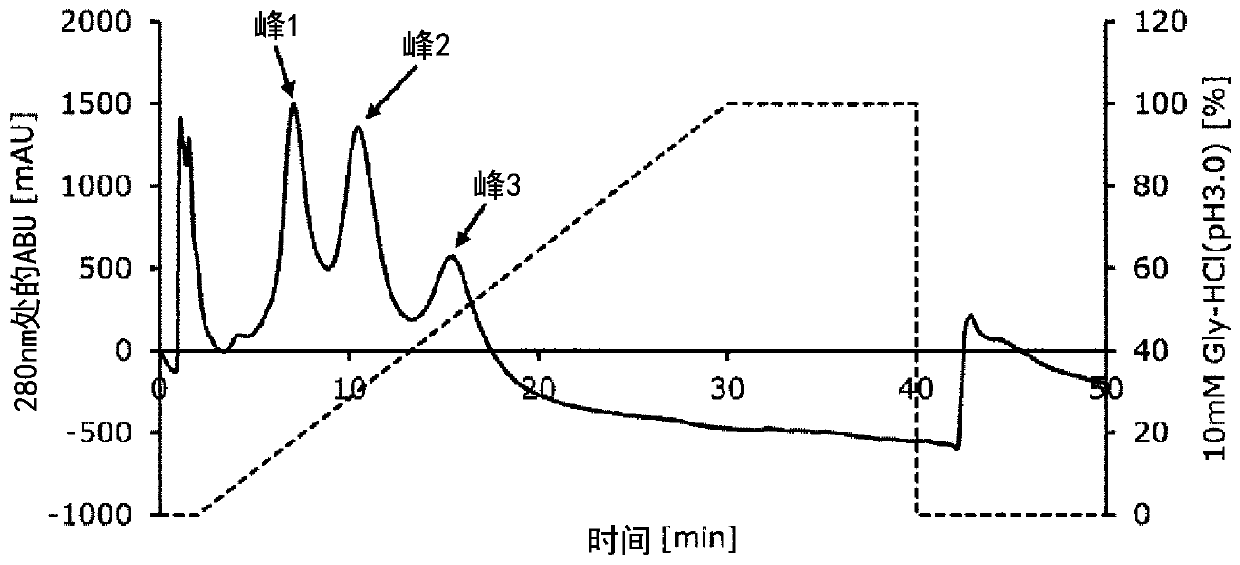
[0083] Example 2 Evaluation of IgG1-binding ability of Fc-binding proteins
[0084] (1) Each transformant expressing the Fc-binding protein produced in Example 1 was cultured in 20 mL of 2YT liquid medium supplemented with 50 μg / mL kanamycin, and cultured with aerobic shaking at 37° C. overnight, Thereby pre-cultivation was carried out respectively.
[0085] (2) Cultivate 10 mL of each pre-culture solution in 1000 mL of 2YT liquid medium (peptone 16 g / L, yeast extract 10 g / L, sodium chloride 5 g / L) supplemented with 50 μg / mL kanamycin, And cultured with aerobic shaking at 37°C overnight.
[0086] (3) The culture temperature was changed to 20° C. 1.5 hours after the start of culture, followed by shaking culture for 30 minutes. Thereafter, isopropyl-β-thiogalactopyranoside (IPTG) was added to a final concentration of 0.01 mM, and aerobic shaking culture was continued overnight at 20°C.
[0087] (4) After the end of the culture, the cells were collected by centrifugation, susp...
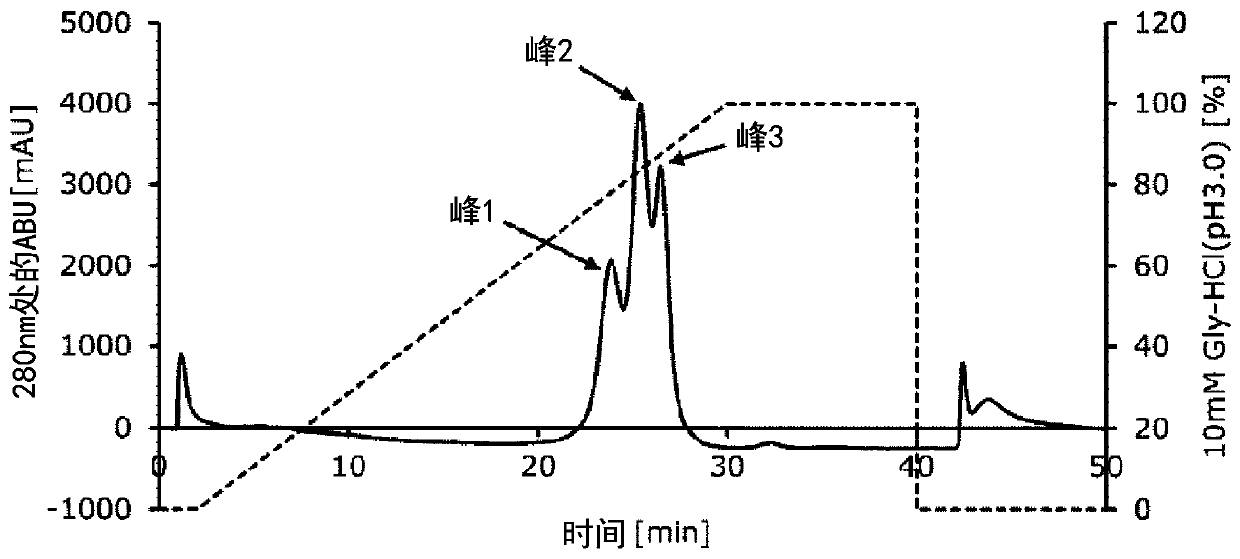
Embodiment 3
[0097] Example 3 Preparation of Fc-binding protein (FcR9_F_Cys) of the present invention with cysteine tag added
[0098] (1) PCR was performed using the expression vector pET-FcR9_F containing the polynucleotide described in SEQ ID NO: 10 encoding the protein composed of the amino acid sequence described in SEQ ID NO: 9 produced in Example 2 as a template. The primers used in PCR were oligonucleotides consisting of the sequences described in SEQ ID NO: 11 (5′-TAGCCATGGGCATGCGTACCGAAGATCTGCCGAAAGC-3′) and SEQ ID NO: 12 (5′-CCCAAGCTTATCCGCAGGTATCGTTGCGGCACCCTTGGGTAATGGTAATATTCACGGTCTCGCTGC-3′). A reaction solution having the composition described in Table 3 was prepared. Thereafter, the reaction solution was heat-treated at 98°C for 5 minutes, and the first step was performed at 98°C for 10 seconds, the second step was performed at 55°C for 5 seconds, and the second step was performed at 72°C for 1 minute. 3 steps, these 3 steps were repeated 30 cycles as one cycle of reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com