Application of JMJD3 inhibitor GSK-J4.HCL in preparing anti-colitis drugs
A colitis and inflammation-inhibiting technology, applied in the field of medicine, can solve problems such as colitis, which is rarely involved in the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0020] Embodiment 1 BMDMs separation and purification and induction culture
[0021] Sterilized surgical instruments: large scissors, small scissors, small forceps, 200-mesh screen. And prepare 1mL sterile syringe, 1640 culture medium, Gibco serum. Take 4 male C57BL / 6J mice of about 25g, kill them by cervical dislocation, soak in 75% alcohol for 3-5min. Use scissors and tweezers to carefully peel off the skin and muscles of the mouse legs, cut off the hip joint to the ankle leg bone, and soak in 75% alcohol for 3 minutes. Then place it in a clean large dish filled with cold PBS, carefully cut off the joints at both ends, place the joint cavity in a new large dish containing 1640 culture solution, and repeatedly wash it several times from both ends with a 1mL syringe until the joint cavity turns white , the washing solution was gently blown twice, filtered into a new large dish with a 200-mesh sieve, centrifuged at 1000 rpm for 3 minutes, discarded the supernatant, resuspende...
Embodiment 2
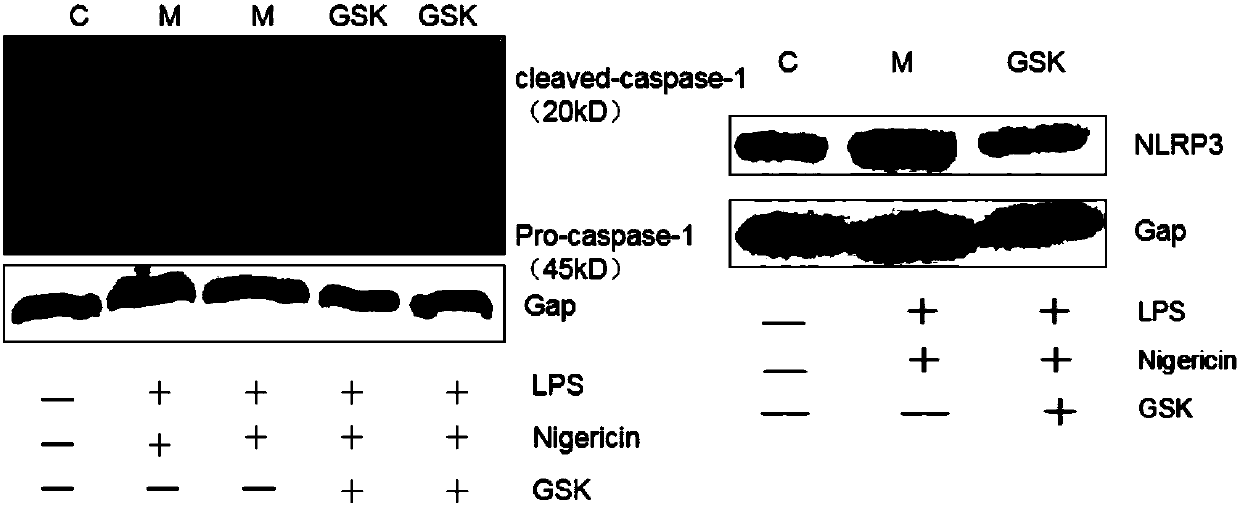
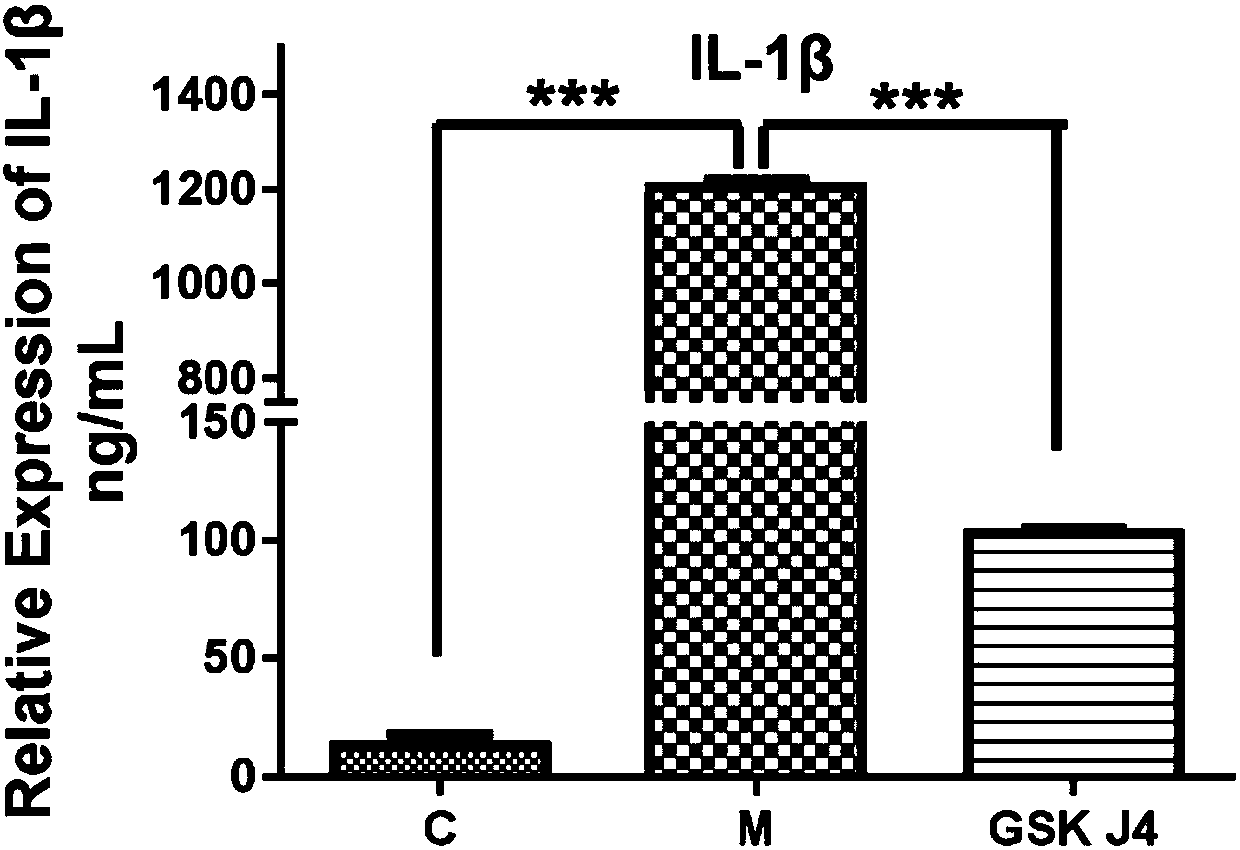
[0022] Inhibition of Example 2GSK-J4 HCL on the inflammatory process of BMDMs stimulated by LPS and Negricin
[0023] Grouping: The synchronized 6cm cell culture dishes inoculated with BMDMs were randomly divided into 3 groups, namely: ① normal control group: no treatment; ② model group: add LPS 200ng / mL, add Negricin 10uM after culturing for 10h, and incubate for 30min. ③GSK-J4·HCL administration group: GSK-J4·HC with a final concentration of 10uM was added 4 hours before the addition of LPS and Negricin. The cell supernatant was collected and the total protein was extracted, and the protein expression of inflammasome NLRP3, ASC, Cleavaged-caspase-1 and the secretion level of related inflammatory factors were detected by western blot and ELISA. The experimental data were analyzed by one-way analysis of variance, p<0.05, the results showed that GSK-J4·HCL can inhibit the expression of inflammasome NLRP3, ASC, Cleavaged-caspase-1; GSK-J4·HCL can significantly inhibit the expres...
Embodiment 3
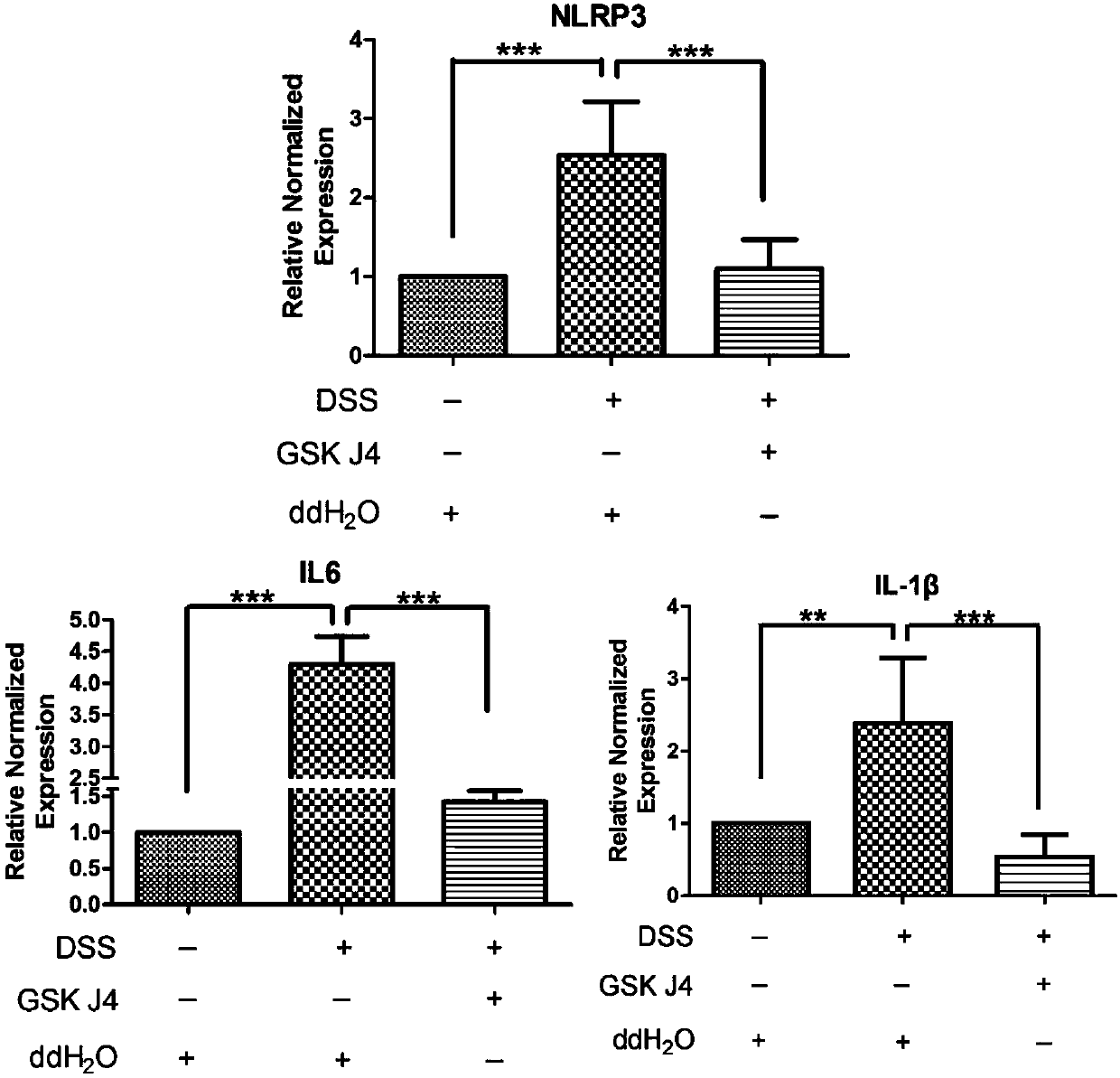
[0025] Example 3 GSK-J4 HCL Therapeutic effect experiment on the mouse Colitis model induced by dextran sulfate sodium salt (DSS)
[0026] Grouping: 7-8 week old male C57BL / 6J mice purchased from Slack Company were randomly divided into 3 groups, namely: ① normal control group; ② model group; ③ GSK-J4·HCL administration group. The preparation of the Colitis mouse model was carried out according to literature (8). Except for the normal group, DSS was prepared into a 3% aqueous solution with sterilized double distilled water, and the mice drank freely. The day of drinking was recorded as the first day, and the newly prepared 3% DSS was replaced every three days. The GSK-J4·HCL administration group was administered orally by intragastric administration on the first day, and each animal was continuously administered at 30 mg / kg / day for 11 days, and the model group was orally administered with an equal volume of normal saline. From the first day, body weight was weighed every day,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com