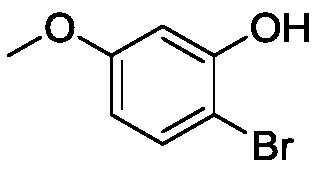
Synthesis method of 2-bromo-5-methoxyphenol
A technology of methoxyphenol and synthesis method, which is applied in the field of synthesis of chemical intermediates - 2-bromo-5-methoxyphenol, can solve problems such as difficult separation, low yield, and difficult post-processing, and achieve post-processing Simple treatment, high conversion rate, and the effect of reducing the formation of isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
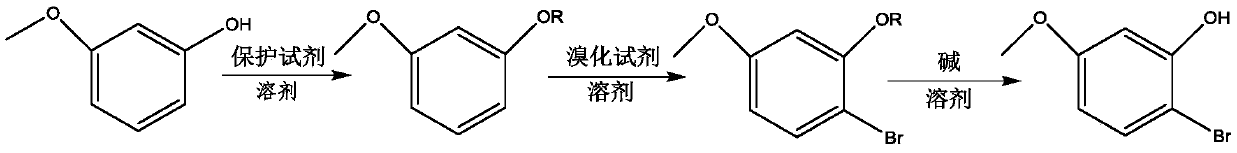
[0047] Embodiment 1, a kind of preparation method of 2-bromo-5-methoxyphenol, take 3-methoxyphenol as raw material, carry out the following steps successively:
[0048] 1), 1.00g (8.06mmol) of 3-methoxyphenol, 4.0mL N,N-dimethylformamide (analytical grade) and 1.46g (9.67mmol) of tert-butyldimethylsilyl chloride were put into 50mL In a three-necked flask; under magnetic stirring, 0.87 g (12.80 mmol) of imidazole was added; at room temperature, magnetic stirring was performed for 1 h.
[0049] After the reaction, use saturated sodium bicarbonate solution (about 15.0 mL) to adjust the pH of the reaction solution to 8, then extract with ethyl acetate (30.0 mL), and add anhydrous sodium sulfate (about 5 g) to the resulting extraction phase to dry After filtration, the resulting filtrate was distilled off under reduced pressure (pressure of -0.1MPa) to remove the extractant (ethyl acetate) to obtain the intermediate 1-[[(1,1-dimethylethyl)dimethylsilyl] Oxy]-3-methoxybenzene 1.85 ...
Embodiment 2
[0057] Embodiment 2, a kind of preparation method of 2-bromo-5-methoxyphenol, take 3-methoxyphenol as raw material, carry out the following steps successively:
[0058] 1), 1.00g (8.06mmol) of 3-methoxyphenol, 7.0mL N,N-dimethylformamide (analytical grade) and 1.82g (12.09mmol) of tert-butyldimethylsilyl chloride were put into 50mL In a three-necked flask; 0.87 g (12.80 mmol) of imidazole was added under magnetic stirring; at room temperature, magnetic stirring was carried out for 2 hours.
[0059] The post-treatment after the reaction is the same as step 1) of Example 1; 1.88 g of the intermediate (same as Example 1) was obtained, with a yield of 97.9%.
[0060] 2), put 1.88g (7.89mmol) of all intermediates obtained in step 1) into a 50mL three-necked flask, then add 15.0mL of dichloromethane into the flask, and finally put N-bromosuccinyl in batches under magnetic stirring Imine 1.38g (7.73mmol); magnetic stirring reaction at room temperature for 3h.
[0061] The post-trea...
Embodiment 3
[0064] Embodiment 3, a kind of preparation method of 2-bromo-5-methoxyphenol, take 3-methoxyphenol as raw material, carry out the following steps successively:
[0065] 1) Put 1.00g (8.06mmol) of 3-methoxyphenol, 4.0mL cyclohexane (analytical grade) and 0.76g (9.68mmol) of acetyl chloride into a 50mL three-necked flask; at room temperature, magnetically stir the reaction for 3h.
[0066] For post-processing after the reaction, refer to step 1) of Example 1; 1.29 g of intermediate 3-methoxyphenyl acetate was obtained, with a yield of 96.6%.
[0067] 2), 1.29g (7.79mmol) of all the intermediate 3-methoxyphenylacetate obtained in step 1) was dropped into a 50mL three-necked flask, then 12.9mL of acetonitrile was added to the flask, and finally N was added in batches under magnetic stirring. -Bromosuccinimide 1.36g (7.63mmol); at 40°C, react with magnetic stirring for 4h.
[0068] For post-processing after the reaction, refer to step 2) of Example 1; 1.71 g of 2-bromo-5-methoxyph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com