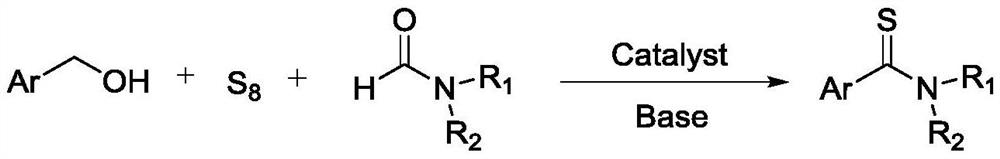Method for preparing aryl thioamide compound
An arylthioamide and compound technology, applied in the field of organic synthesis, can solve the problems of harsh reaction conditions of substrates, poor universality of substrates, unstable Lawson's reagent, etc., to improve atom economy and protect the environment. Operator health, the effect of ensuring operator health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, a kind of preparation method of aryl thioamide compound, take benzyl alcohol as raw material:
[0042] Under nitrogen protection, benzyl alcohol (0.43g, 3.9mmol), sublimed sulfur (0.25g, 7.8mmol), sodium hydroxide (0.31g, 7.8mmol) were added to 2.2mL N,N-dimethylformamide, Then cesium chloride (0.13g, 0.78mmol) and ethylenediaminetetraacetic acid (0.23g, 0.78mmol) were added, and the reaction was stirred for 6 hours at a reaction temperature of 60°C.
[0043] After the reaction was completed, 2.2 mL of deionized water was added to the resulting reaction liquid to quench the reaction liquid, followed by extraction with ethyl acetate (2.2 mL × 3) to obtain an aqueous phase and an organic phase respectively; then saturated saline (2.2 mL ×3) Wash the organic phase, and dry it with anhydrous sodium sulfate, filter (remove sodium sulfate), concentrate by rotary evaporation (remove ethyl acetate), purify by silica gel (100-200 mesh) column chromatography, and us...
Embodiment 2
[0044] Embodiment 2, a kind of preparation method of aryl thioamide compound, take benzyl alcohol as raw material:
[0045]Under nitrogen protection, benzyl alcohol (0.43g, 3.9mmol), sublimed sulfur (0.25g, 7.8mmol), potassium hydroxide (0.35g, 6.2mmol) were added to 3.0mL formamide, and cesium bromide (0.17 g, 0.78mmol) and salicylaldehyde (0.10g, 0.78mmol), stirred and reacted for 12 hours at room temperature.
[0046] After the reaction was completed, 3.0 mL of deionized water was added to the resulting reaction liquid to quench the reaction liquid, followed by extraction with ethyl acetate (3.0 mL×3) to obtain an aqueous phase and an organic phase, respectively, and then saturated saline (3.0 mL ×3) The organic phase was washed, dried with anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, purified by silica gel (100-200 mesh) column chromatography, and purified with a mixture of ethyl acetate / petroleum ether (1:12 volume ratio) As an eluent, the amou...
Embodiment 3
[0047] Embodiment 3, a kind of preparation method of aryl thioamide compound, take benzyl alcohol as raw material:
[0048] Under nitrogen protection, benzyl alcohol (0.43g, 3.9mmol), sublimed sulfur (0.20g, 6.2mmol), cesium hydroxide (0.70g, 4.7mmol) were added to 4.0mL N-methylformamide, and then chlorine Potassium chloride (0.15 g, 2.0 mmol) and salicylaldehyde (0.24 g, 2.0 mmol) were reacted with stirring at a reaction temperature of 50° C. for 8 hours.
[0049] After the reaction was completed, 4.0 mL of deionized water was added to the resulting reaction liquid to quench the reaction liquid, followed by extraction with ethyl acetate (4.0 mL×3) to obtain an aqueous phase and an organic phase, respectively, and then saturated saline (4.0 mL ×3) The organic phase was washed, dried with anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, purified by silica gel (100-200 mesh) column chromatography, and purified with a mixture of ethyl acetate / petroleum eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com