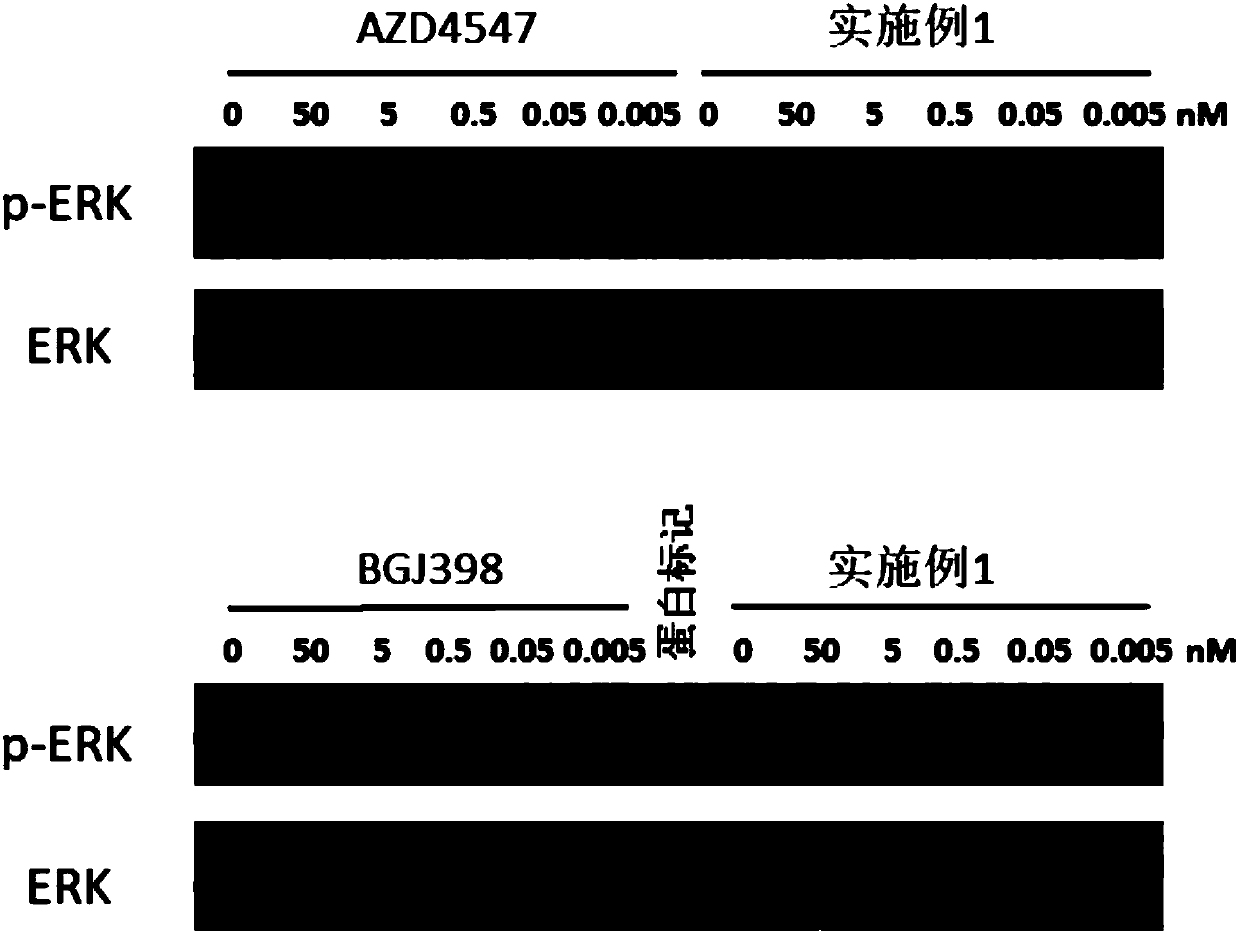
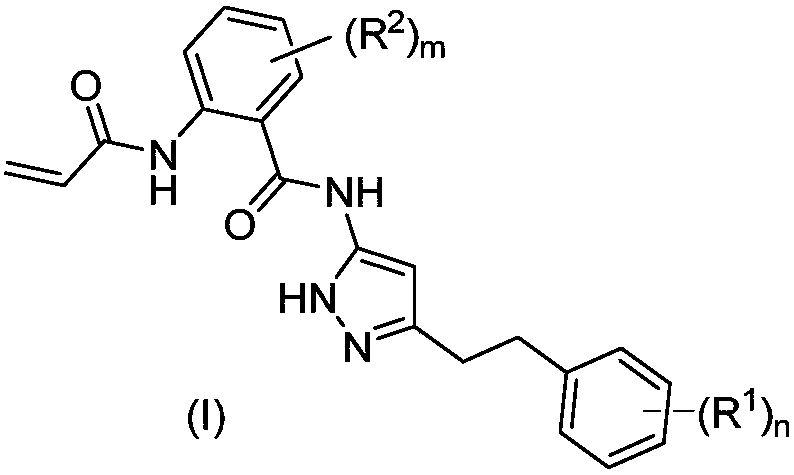
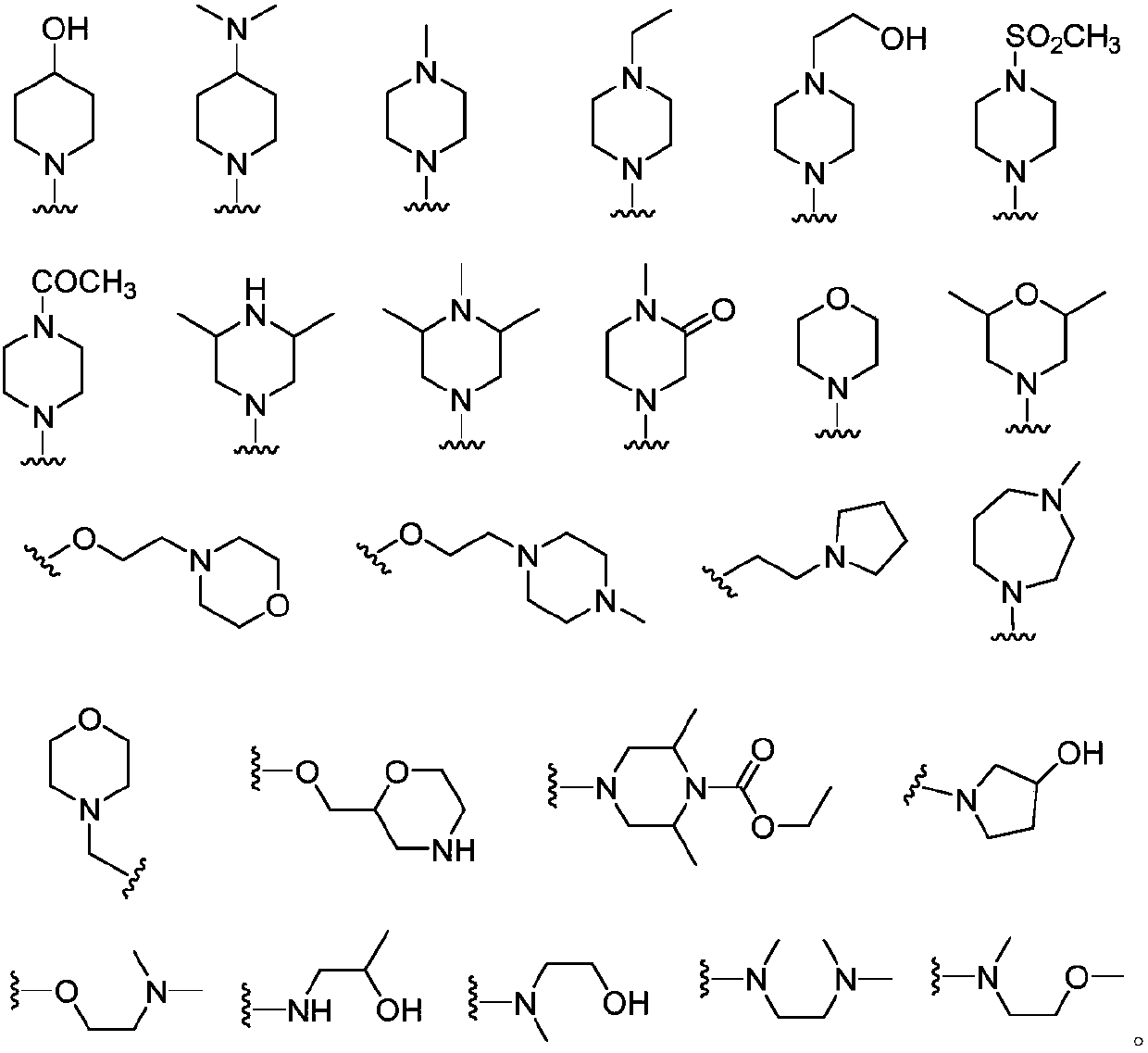
Amide group pyrazol compound used as FGFR irreversible inhibitor
A group, cyano group technology, applied in the field of new pyrazole derivatives, can solve the problems of weak inhibitory effect, insignificant inhibitory effect, reduced tumor inhibitory effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Example 1: 2-acrylamido-N-(3-(3,5-dimethoxyphenethyl)-1H-pyrazol-5-yl)benzamide
[0221]
[0222] Put 76mg (0.31mmol) intermediate A in the round bottom flask, N 2 Add 2ml of dry toluene under protection to dissolve, stir in ice bath for 10min, then add dropwise 0.29ml of trimethylaluminum solution (1.6M, 0.46mmol), react for 1h, add 100mg (0.46mmol) of intermediate B, and stir for 10min Remove the ice bath, react at 60°C for 18h, cool down, add H 2 O quenched the reaction, extracted with ethyl acetate, and the resulting solution was washed with saturated NaCl solution, anhydrous NaCl 2 SO 4 dry. Separation and purification by silica gel column chromatography (gradient elution, dichloromethane:methanol=200:1 to 70:1) to obtain white powdery solid 2-acrylamido-N-(3-(3,5-dimethoxy Phenylethyl)-1H-pyrazol-5-yl)benzamide (Example 1, 67 mg, 52%). 1 H NMR(400MHz,DMSO)δ12.24(s,1H),11.09(s,1H),10.89(s,1H),8.34(d,J=8.3Hz,1H),7.86(d,J=7.8Hz ,1H),7.53(t,J=7.8Hz,1H),7.19(t...
Embodiment 2
[0223] Example 2: 2-acrylamido-N-(5-(3,5-dimethoxyphenethyl)-1H-pyrazol-3-yl)-4-((2-(dimethylamino) Ethyl)(methyl)amino)benzamide
[0224]
[0225] Add 150mg compound A (0.607mmol) in two-necked bottle, N2 After being dissolved by adding 2.2ml of ultra-dry toluene under protection, it was stirred under ice bath for a while, and then 1.3ml of trimethylaluminum solution (1.6M, 2.12mmol) was added slowly, and stirred for another hour under ice bath. One hour later, 194mg of compound C (0.607mmol) was added, and then the reaction system was moved to an oil bath at 110°C to continue stirring. After 10 hours of reaction, the plate was spotted, and the reaction was completed. Add 10 ml of water to the reaction solution to quench the reaction solution, extract with ethyl acetate, wash with saturated brine, and dry over anhydrous sodium sulfate. Add silica gel to make sand, separate and purify on silica gel column (gradient elution from DCM:MeOH=100:1 to DCM:MeOH=10:1) to obtain m...
Embodiment 3
[0226] Example 3: 2-acrylamido-N-(3-(3,5 dimethoxyphenethyl)-1H-pyrazol-5-yl)-4-((3R,5S)-3,5 -Dimethylpiperazin-1-yl)benzamide
[0227]
[0228] Add compound A (745mg) into 20mL toluene under nitrogen protection, stir for a while under ice-water bath, slowly add 2M toluene solution (4.5ml) of trimethylaluminum, keep stirring in ice-water bath for 0.5-1 hour, add compound D ( 1.3g), the reaction system was transferred to an oil bath and heated to reflux (110-115°C), reacted for 5 hours, LCMS detected that the reaction was complete, the reaction solution was poured into 30mL water to quench, extracted with ethyl acetate, and the organic phase was washed with saturated brine, After drying with anhydrous sodium sulfate, the solvent was spin-dried, and then prepared and lyophilized to obtain a white solid 2-acrylamido-N-(3-(3,5-dimethoxyphenethyl)-1H-pyrazole-5 -yl)-4-((3R,5S)-3,5-dimethylpiperazin-1-yl)benzamide (Example 3, 48 mg, 3%). 1 H NMR (400MHz, DMSO-d6)δ12.04(s,1H),10...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap