Preparation method of Brivaracetam intermediate
A technology of chiral intermediates and equations, applied in the medical field, can solve problems such as unstable chemical properties, difficult to purchase, and expensive, and achieve the effect of optical purity and easier purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
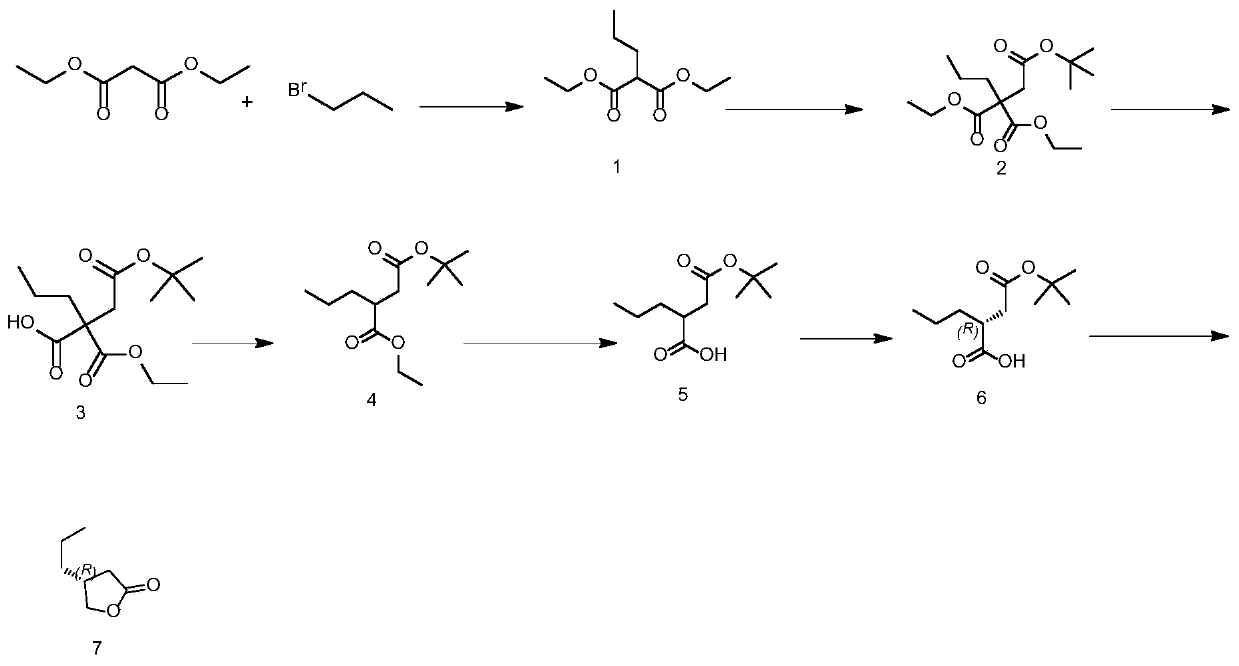
[0047] Such as figure 1 Shown:
[0048] Preparation of the compound of formula (1): Add N,N-dimethylformamide into the reaction bottle, start stirring, add 0.662 mmol of potassium hydroxide, and lower the temperature in the bottle to 0°C. Add 0.662 mmol of diethyl malonate, add 0.662 mmol of bromopropane, and keep warm at 0°C for 4 to 6 hours. After the reaction, slowly add 400 g of tap water and 360 g of ethyl acetate to the reaction solution and stir for 30 minutes. Stand still and separate to obtain an organic layer. Concentrate the organic layer under reduced pressure to dryness to obtain the compound of formula (1).
[0049] Preparation of the compound of formula (2): Add N,N-dimethylformamide into the reaction flask, start stirring and add 0.494 mmol of potassium hydroxide, after the addition is complete, cool down to 0°C, add 0.494 mmol of the compound of formula (1), add 0.494 Mmol tert-butyl bromoacetate, keep warm at 0°C for 2-3 hours. After the reaction, slowly add...
Embodiment 2
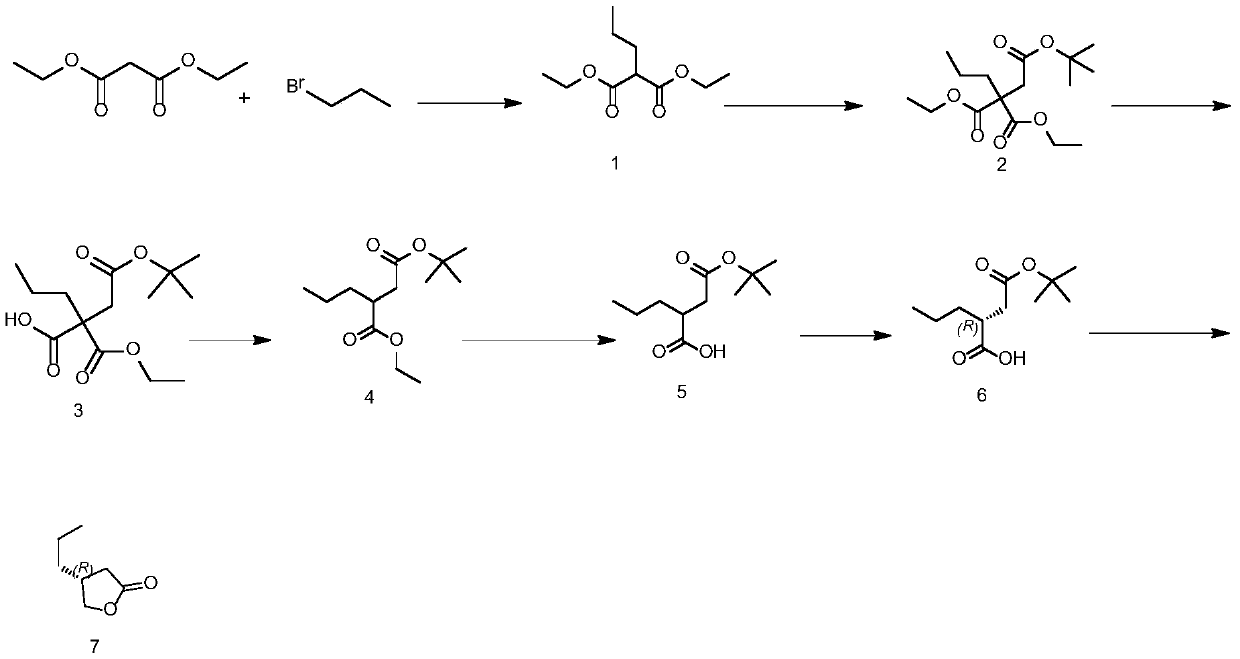
[0056] Such as figure 1 Shown:
[0057] Preparation of the compound of formula (1): Add dimethyl sulfoxide into the reaction bottle, start stirring, add 1.324 mmol potassium carbonate, and lower the temperature inside the bottle to 45°C. Add 0.662 mmol of diethyl malonate, add 1.324 mmol of bromopropane, and keep warm at 45° C. for 4 to 6 hours. After the reaction, slowly add 400 g of tap water and 360 g of ethyl acetate to the reaction solution and stir for 30 minutes. Stand still and separate to obtain an organic layer. Concentrate the organic layer under reduced pressure to dryness to obtain the compound of formula (1).
[0058] Preparation of the compound of formula (2): Add dimethyl sulfoxide etc. into the reaction flask, start stirring and add 0.988 mmol of sodium carbonate, after the addition is complete, cool down to 45°C, add 0.494 mmol of the compound of formula (1), add 0.988 mmol of For tert-butyl bromoacetate, keep warm at 45 for 2 to 3 hours. After the reactio...
Embodiment 3
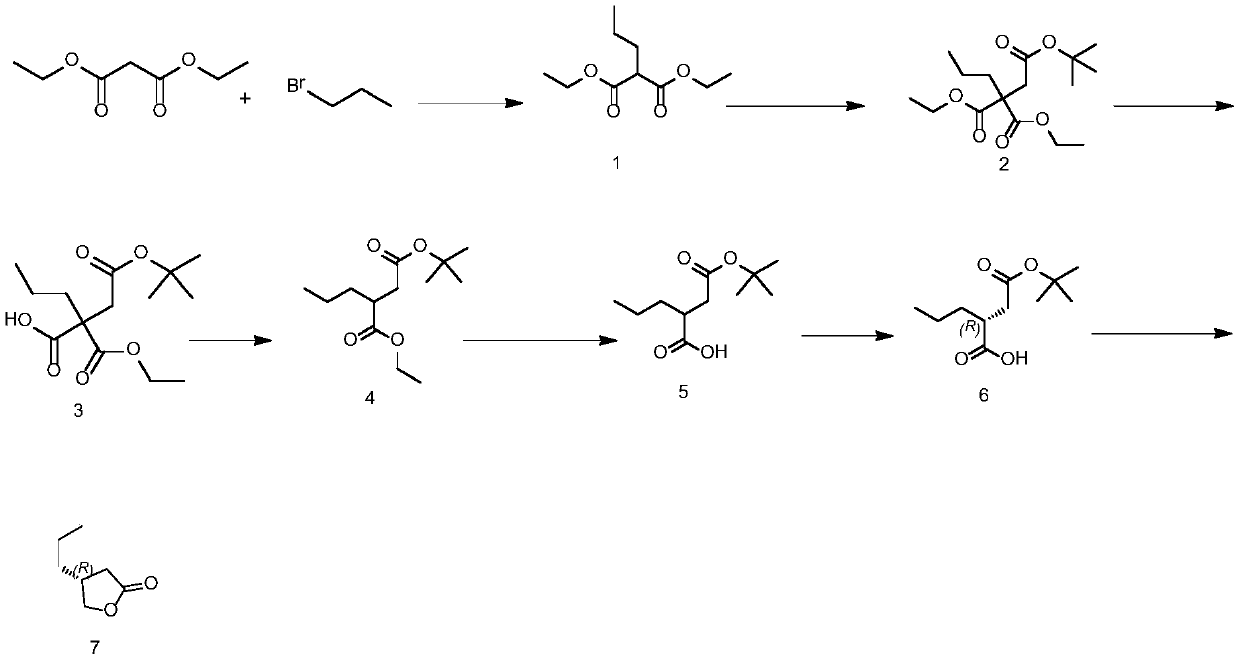
[0065] Such as figure 1 Shown:
[0066] Preparation of the compound of formula (1): add tetrahydrofuran to the reaction bottle, start stirring, add 0.728mmol of sodium hydroxide, lower the temperature in the bottle to 20°C, add 0.662mmol of diethyl malonate, add 0.728mmol of bromopropane , keep warm at 20°C and react for 4-6 hours. After the reaction, slowly add 400 g of tap water and 360 g of ethyl acetate to the reaction solution and stir for 30 minutes. Stand still and separate to obtain an organic layer. Concentrate the organic layer under reduced pressure to dryness to obtain the compound of formula (1).
[0067] Preparation of the compound of formula (2): Add tetrahydrofuran into the reaction flask, start stirring and add 0.543 mmol of sodium hydroxide, after the addition is complete, cool down to 20°C, add 0.494 mmol of compound of formula (1), and add 0.543 mmol of tert-butyl bromoacetate Ester, keep warm at 20°C for 2-3 hours. After the reaction, slowly add 600 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com