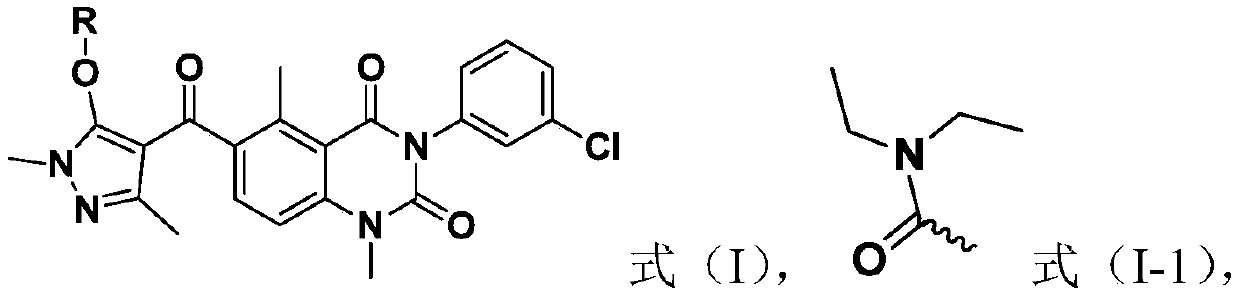
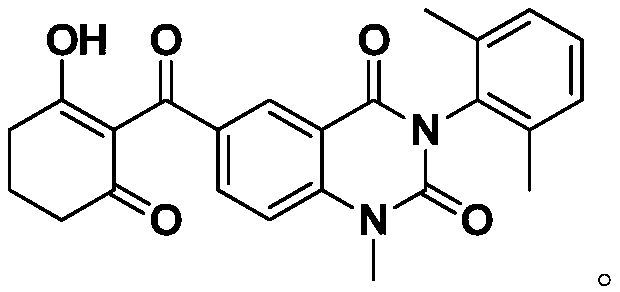
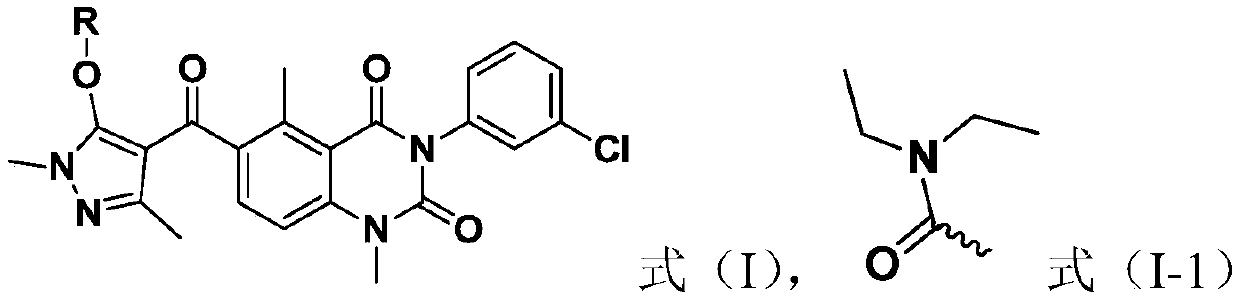
Quinazolinedione compound and applications thereof, and pesticide herbicide
A technology of quinazolinediones and compounds, which is applied in the field of pesticides and herbicides, can solve the problems of not providing the biological activity test results of compounds, etc., and achieve the effects of excellent crop safety, subsequent crop safety, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0042] Preparation Example 1: Compound 1 was synthesized using the following synthetic route
[0043]
[0044] Preparation of compound 2a: Dissolve 2-methyl-6-aminobenzoic acid (i.e., compound 1a) (100mmol) in acetic acid (1200ml), add 1mol / L ICl acetic acid solution dropwise under stirring, and continue stirring after the addition is complete Reaction 2h. A large amount of solids will be precipitated during the reaction, and TLC will track the reaction process, after the reaction is completed. The filtrate was removed by suction filtration under reduced pressure, the obtained solid was washed twice with 500 mL of acetic acid, and after drying, an off-white solid was obtained with a yield of 93%.
[0045] Preparation of compound 3a: under nitrogen protection, compound 2a (20 mmol) and 30 ml of pyridine were added into a 100 mL two-necked bottle. After stirring until the solid was completely dissolved, m-chlorophenylisocyanate (25 mmol) was slowly added to the reaction sys...
preparation example 2
[0052] Preparation example 2: the preparation method of compound 2
[0053] Compound 1 (91.3mmol) was dissolved in THF (457ml), and triethylamine (182.7mmol), N,N-diethylformyl chloride (182.7mmol) and DMAP (27.4mmol) were added in sequence. Raise the temperature to reflux for 5 hours. After the reaction is completed, cool to return to room temperature, and stand at 0°C for 2 hours. The solid is removed by suction filtration. Methyl chloride was dissolved and the organic phase of dichloromethane was washed twice with water, the solvent was distilled off under reduced pressure, and the obtained solid was recrystallized with diethyl ether, with a yield of 91%.
[0054] For the preparation methods of the compounds in the above reference samples, refer to the aforementioned Preparation Example 1 and Preparation Example 2.
[0055] The characterization data of the above-mentioned reference sample and the compound of the present invention are listed in Table 1 respectively:
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com