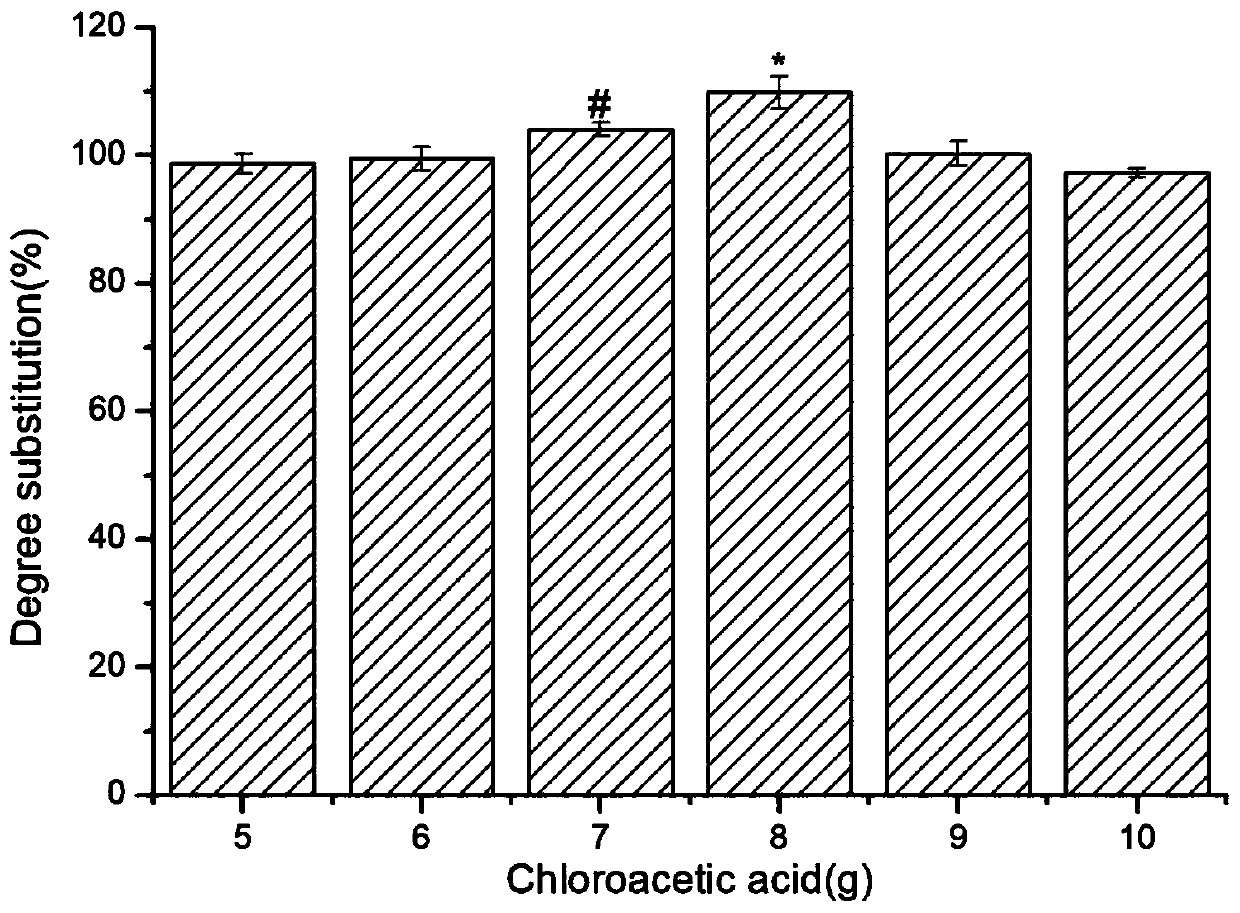
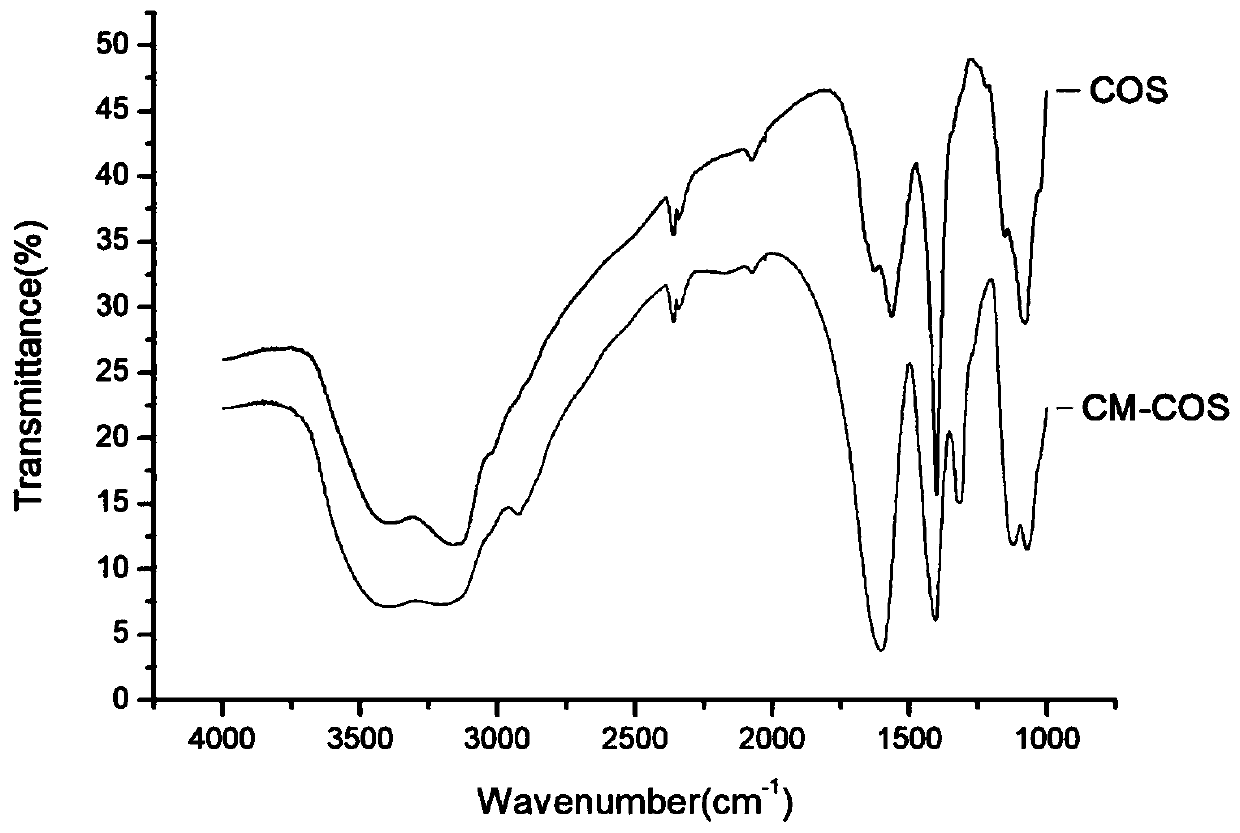
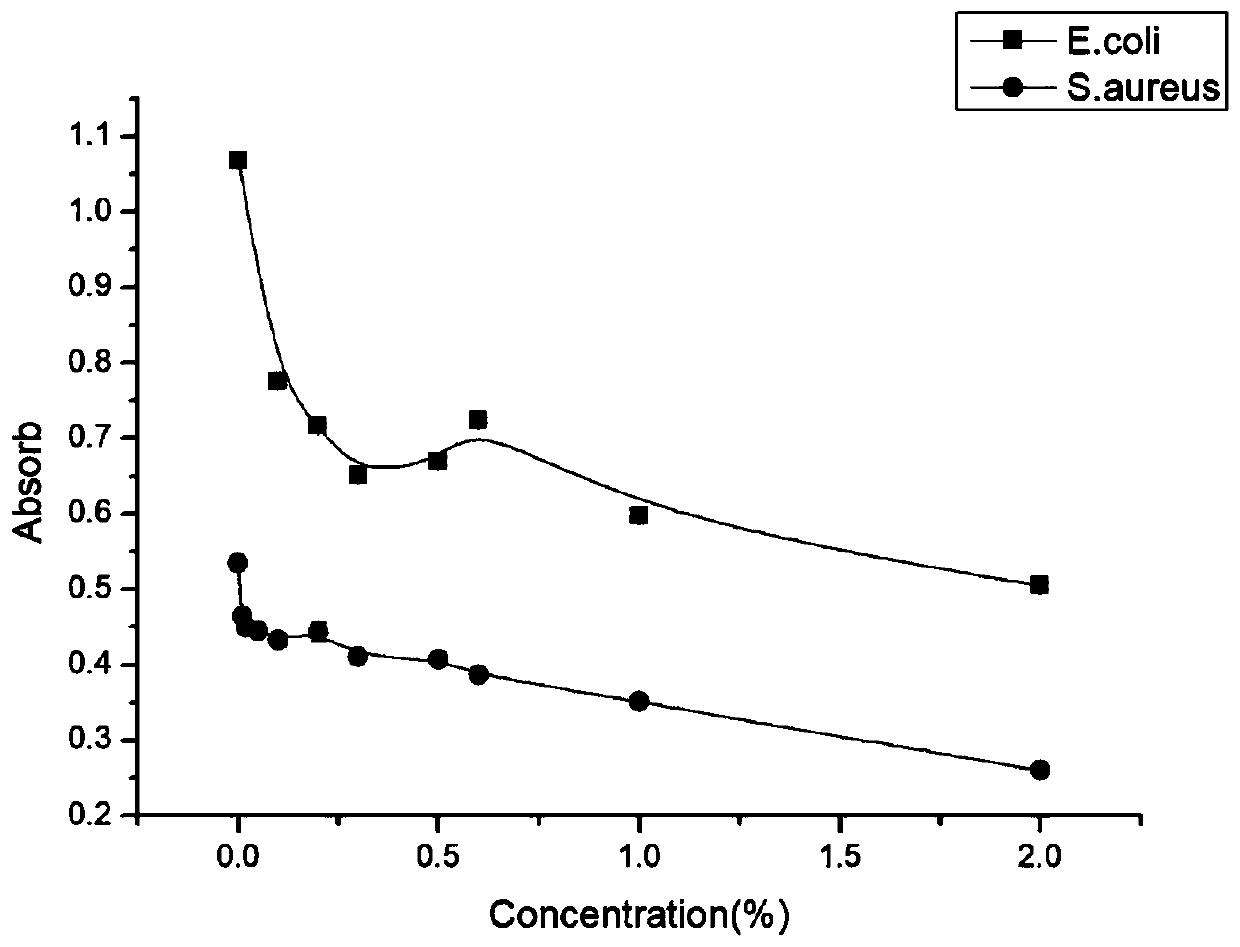
Preparation method of carboxymethyl chito-oligosaccharide
A technology of carboxymethyl chitosan oligosaccharide and chitosan oligosaccharide is applied in the field of preparation of carboxymethyl chitosan oligosaccharide, which can solve the problems of immature carboxymethyl chitosan oligosaccharide technology, unoptimized process and parameters, etc., and achieve excellent antibacterial properties. Antioxidant activity, reduced preparation time, no pollution residue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The technical scheme adopted in the present invention is, a kind of preparation method of carboxymethyl chitosan oligosaccharide, comprises the following steps:
[0031] Step 1, add chitosan oligosaccharide into isopropanol and shake at room temperature to obtain swelling chitosan oligosaccharide; the ratio of chitosan oligosaccharide to isopropanol is 2g: 15ml~25ml, wherein, chitosan oligosaccharide is calculated by mass, and isopropanol is calculated by mass volume calculation;
[0032] Step 2, add NaOH solution to the swelling chitosan oligosaccharide obtained in step 1, and oscillate at room temperature to obtain an alkaline feed liquid; the mass percentage concentration of the NaOH solution is 30%, and the alkalization time is 2h to 12h; the chitosan oligosaccharide and NaOH solution The ratio is 2g: 6.67ml ~ 20ml, wherein, chitosan oligosaccharide is calculated by mass, and NaOH solution is calculated by volume.
[0033] Step 3, add isopropanol solution of chloro...
Embodiment 1
[0041] Step 1, weigh 2g of chitosan oligosaccharide into a 1L beaker, stick a plastic wrap, add 15ml of isopropanol, swell at room temperature for 30min;
[0042] Step 2, first add 6.67ml of NaOH solution with a mass percentage concentration of 30% to the swollen chitosan oligosaccharide obtained in step 1, and then shake and alkalize at room temperature for 2 hours to obtain an alkaline feed solution;
[0043] Step 3, add isopropanol solution of chloroacetic acid to the alkaline feed solution obtained in step 2 for carboxylation reaction at 35°C for 4h, wherein the quality of chloroacetic acid in the isopropanol solution of chloroacetic acid is 2g, and the volume of isopropanol 50ml;
[0044] Step 4, adjust the pH of the feed solution after carboxylation in step 3 to neutral with 9.5% HCl by mass percentage, then ethanol alcohol precipitation for 6.5 hours, pour off the supernatant, and dissolve the precipitate at the bottom of the cup in an appropriate amount of Ethanol alc...
Embodiment 2
[0047] The implementation steps are the same as in Example 1 except that the alkalization time, the amount of chloroacetic acid added, and the carboxylation time are different, and the rest of the steps are the same. The alkalization time in this example is 7h, the amount of chloroacetic acid added is 3g, and the carboxylation time is 12h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com