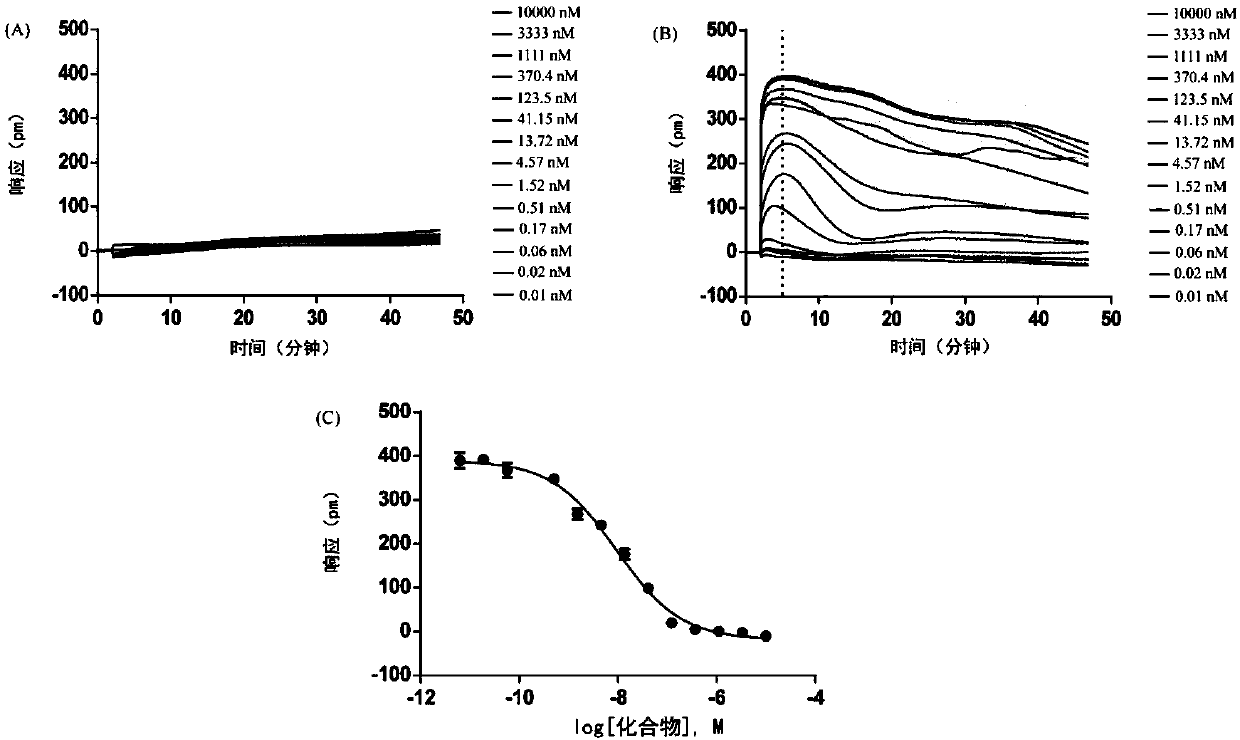
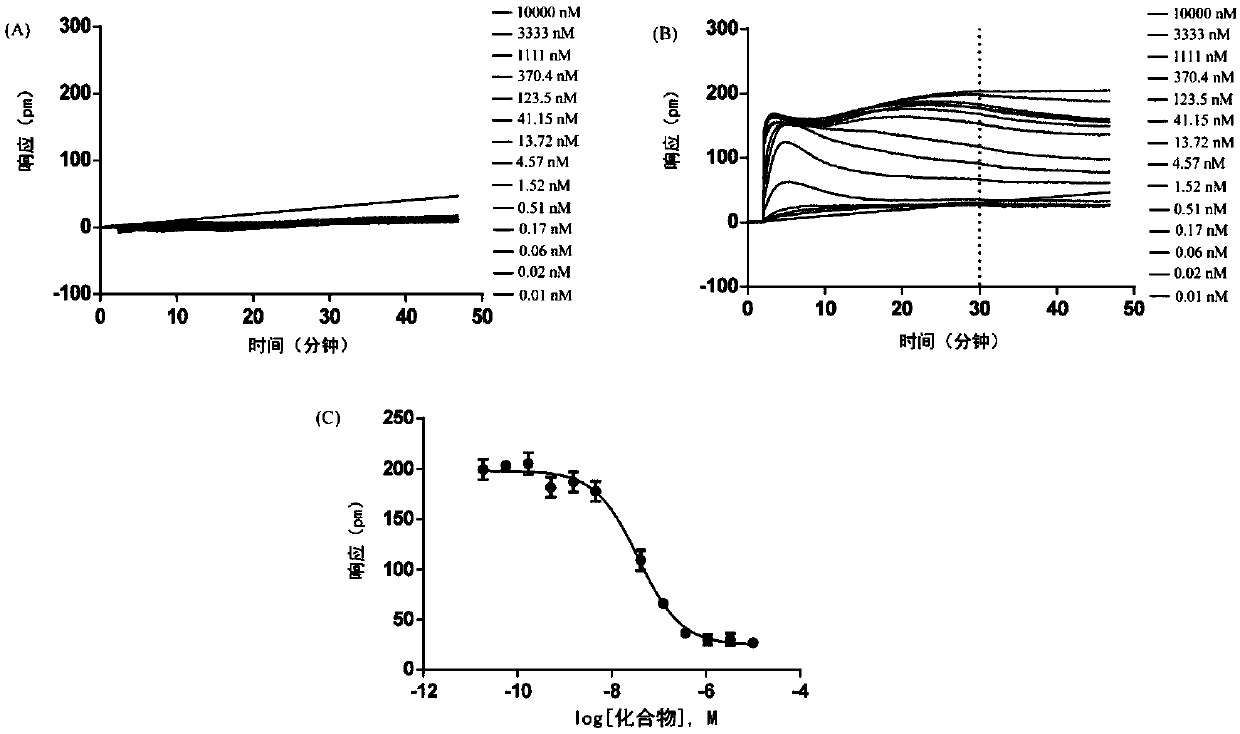
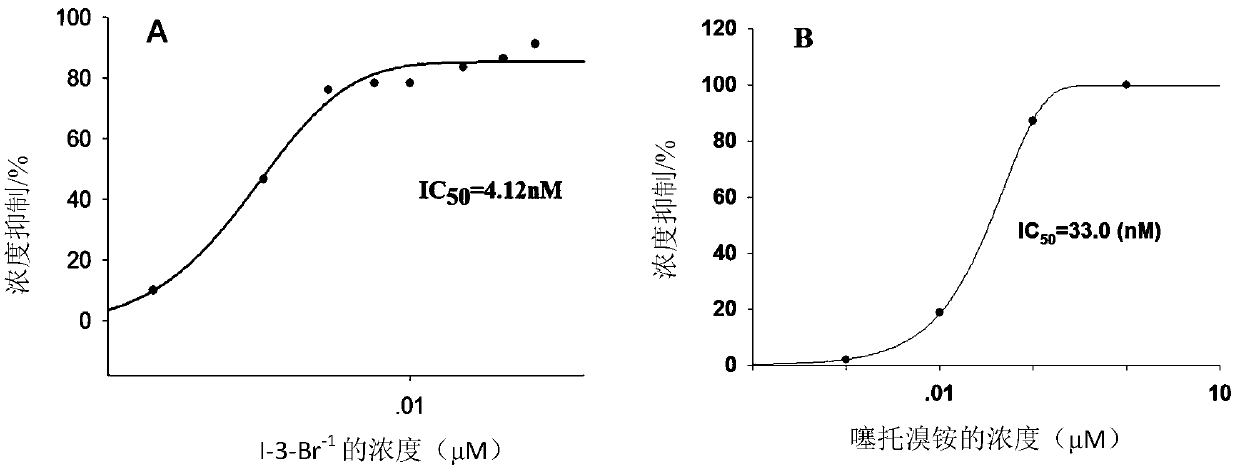
2-(2,2-diarylethyl)-cyclamine derivative or salt, and synthesis and application and composition thereof
A technology of derivatives and cyclic amines, applied in the field of 2--cyclic amine derivatives and its preparation, to achieve the effect of enhancing antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] 2-(2,2-Diphenylethyl)-N-methylpiperidine (II-1)
[0240] synthetic route:
[0241]
[0242] 1.1 Synthesis of 2-(2,2-distyryl)-N-methylpiperidine
[0243] Weigh 2-bromo-1,1-diphenylethylene (2.59g, 10mmol) and dissolve it in 60ml N-methylpiperidine, add MnCl 2 (126 mg, 1 mmol), stirred at room temperature. Slowly add 40ml of dimethyl zinc in n-hexane solution (1.0M, 40mmol), heat to 50-100°C (usually 70°C), stir the reaction, monitor the reaction by TLC, after the reaction is complete. The reaction was quenched with 20-100ml saturated sodium hydroxide solution, the mixture was extracted with dichloromethane (3x 100ml), the organic phases were combined, dried with anhydrous sodium sulfate, and after the solvent was removed by rotary evaporation, the resulting residue was subjected to silica column chromatography After separation and purification, 1.9 g of a light brown oily product can be obtained (69% yield). 1 HNMR (400MHz, CDCl 3 ), δ: 7.43-7.34(m, 3H), 7.31-7.24...
Embodiment 2
[0247] 2-[2-(2-Hydroxy-phenyl)-phenethyl]-N-methylpiperidine (II-2, and its chiral monomers II-2a-1 and II-2a-2), 2 -[2-(2-Hydroxy-phenyl)-phenethyl]-N,N-dimethylpiperidinium bromide (I-2a-Br, and its chiral monomers I-2a-1-Br and I-2a-2-Br) and 2-[2-(2-hydroxy-phenyl)-phenethyl]-N,N-dimethylpiperidinium iodide (I-2a-I)
[0248] synthetic route:
[0249]
[0250] 2.1 Synthesis of (2-methoxymethoxy-phenyl)(phenyl)-methanone
[0251] Dissolve (2-hydroxy-phenyl)(phenyl)-methanone (1.98g, 10mmol) in dichloromethane (50ml), add diisopropylethylamine (30mmol), stir at room temperature, add methyl chloride methyl ether (20 mmol). Heated to reflux for 12 hours, monitored by TLC, after the reaction was completed. The reaction solution was cooled to room temperature, the solvent was removed by rotary evaporation, and purified by silica column chromatography to obtain 2.2 g of a brown oily product of (2-methoxymethoxy-phenyl) (phenyl)-methanone (yield 91 %). 1 H NMR (400MHz, CDC...
Embodiment 3
[0270] 2-[2,2-bis(2-hydroxy-phenyl)-ethyl]-N-methylpiperidine (II-3 racemate, and its chiral monomer II-3-1, II-3 -2), 2-[2,2-bis(2-hydroxy-phenyl)-ethyl]-N,N-dimethylpiperidinium bromide (I-3-Br, and its chiral monomer I -3-1-Br, I-3-2-Br) and 2-[2,2-bis(2-hydroxy-phenyl)-ethyl]-N,N-dimethylpiperidinium iodide (I -3-I, and its chiral monomer I-3-1-I, I-3-2-I)
[0271] synthetic route:
[0272]
[0273] 3.1 Synthesis of bis(2-methoxymethoxy-phenyl)-methanone
[0274] According to the steps described in 2.1 of Example 2, bis(2-hydroxy-phenyl)-methanone is used as raw material, and the amount of DIEA and chloromethyl methyl ether is doubled, and bis(2-methoxymethyl ether) can be prepared Oxy-phenyl)-methanone as brown oily product, 92% yield. 1 HNMR (400MHz, CDCl 3 ), δ: 7.54-7.52 (m, 2H), 7.43-7.39 (m, 2H), 7.12-7.04 (m, 4H), 4.98 (s, 4H), 3.26 (s, 6H).
[0275] 3.2 Synthesis of 3,3-bis(2-methoxymethoxy-phenyl)-methyl acrylate
[0276] According to the steps described...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com