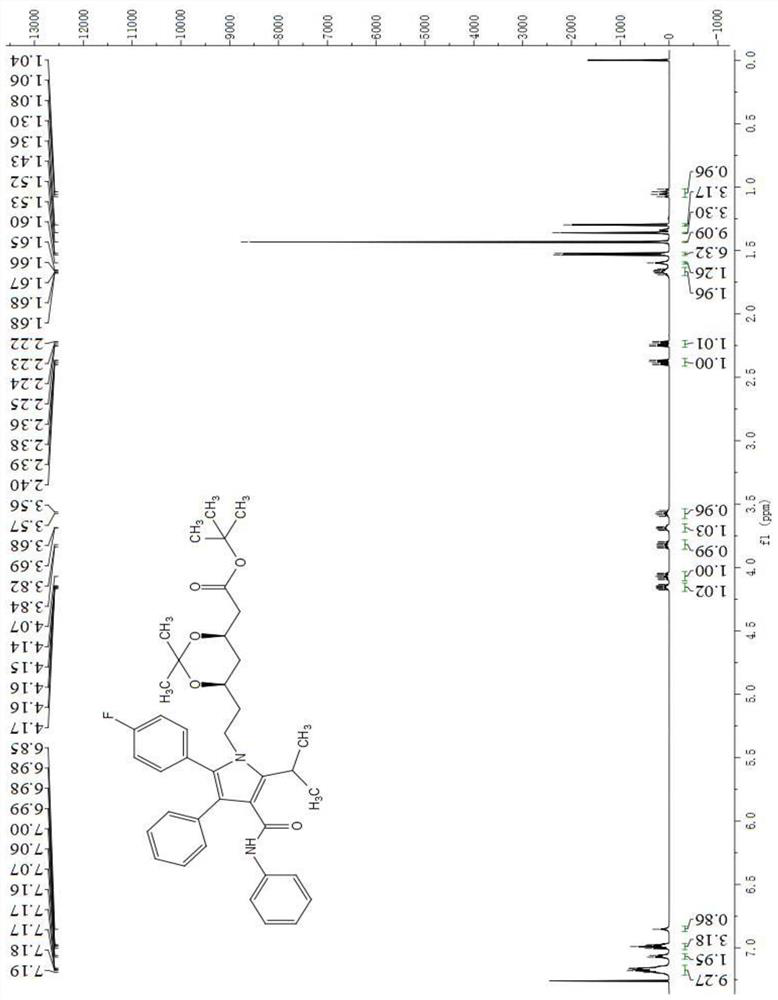
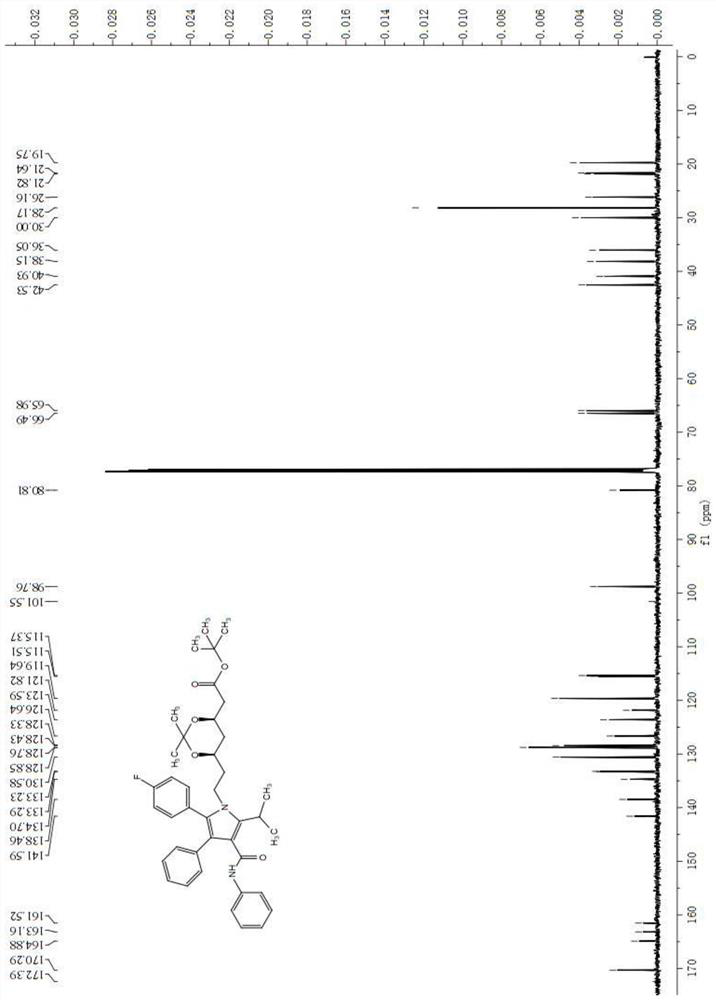
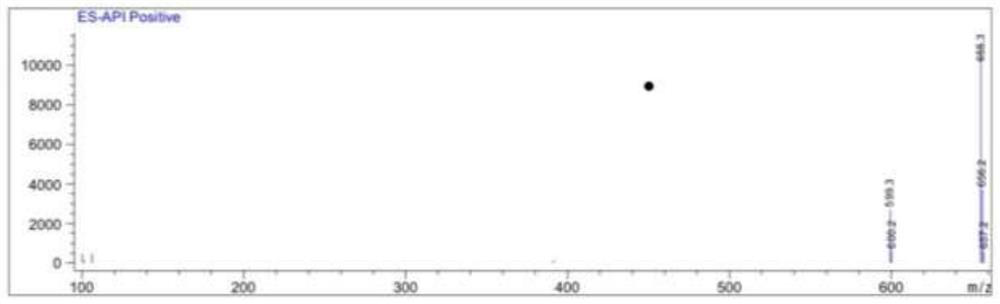
A method for preparing atorvastatin key intermediate l1 by a solvent-free method
An atorvastatin, solvent-free technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of complex post-processing, difficult solvent recovery, long reaction time, etc., and achieve the effect of shortening reaction time, short reaction time and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A method for preparing atorvastatin key intermediate L1 by a solvent-free method, comprising the following steps: adding M4 25g (59.88mmol) to the three-necked reaction flask successively, tetrabutylammonium bisulfate 6.25g (18.41mmol), neopentyl ammonium bisulfate Acid 3.15g (30.84mmol), A9 32.86g (119.76mmol), stirring, heating and melting reaction (external temperature 120°C), using a water separator to separate water, the reaction system is yellowish-brown thick;
[0039] The reaction progress was monitored by HPLC detection method, and the mobile phase conditions were: 75% acetonitrile, and the reaction was complete within 5.5 hours. The raw material M4HPLC content is less than 1%, and the L1 content is greater than 90%;
[0040] After the reaction was complete, the reaction system was cooled to 95°C, ethanol was added to dissolve until clarified, water was added dropwise until turbid, and the temperature was lowered to crystallize to obtain 28.55 g of a light yell...
Embodiment 2
[0046] A method for preparing atorvastatin key intermediate L1 by a solvent-free method, comprising the following steps: adding M4 25g (59.88mmol) to the three-necked reaction flask successively, tetrabutylammonium bisulfate 6.25g (18.41mmol), neopentyl ammonium bisulfate Acid 3.15g (30.84mmol), A9 32.86g (119.76mmol), stirred and heated for melting reaction (external temperature 130°C), water was separated with a water separator, and the reaction system was brown and thick;
[0047] Use HPLC detection method to monitor the reaction process, mobile phase conditions: 75% acetonitrile, 6.5h reaction is complete, raw material M4HPLC content is less than 3%, L1 content is greater than 87%, the reaction is complete;
[0048] After the reaction was complete, the reaction system was cooled to 95°C, 95% ethanol was added to dissolve it, water was added dropwise until cloudy, and the temperature dropped to crystallize to obtain 32 g of a light yellow powdery solid crude product. After t...
Embodiment 3
[0050] A method for preparing atorvastatin key intermediate L1 by a solvent-free method, comprising the following steps: adding M4 25g (59.88mmol) to the three-necked reaction flask successively, tetrabutylammonium bisulfate 6.25g (18.41mmol), neopentyl ammonium bisulfate Acid 3.15g (30.84mmol), A9 19.72g (71.87mmol), stirred and heated for melting reaction (external temperature 120°C), water was separated with a water separator, and the reaction system was brown and thick;
[0051] Adopt HPLC detection method to monitor reaction progress, mobile phase condition: 75% acetonitrile, 7h reaction is complete;
[0052] After the reaction is complete, cool the reaction system to 95°C, add ethanol to dissolve it, add water dropwise until cloudy, cool down and crystallize to obtain 26.67g of a light yellow powder solid crude product, after drying the crude product, recrystallize twice to obtain a white powder Solid 24.59g. The content of the product detected by HPLC was 99.84%; the y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com