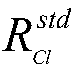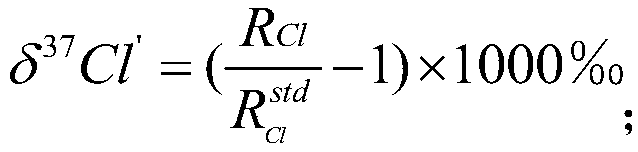Method for purifying and analyzing chlorine isotope in complex matrix sample
A chlorine isotope and complex matrix technology, applied in the analysis of materials, material analysis by electromagnetic means, material separation, etc., can solve problems such as interference, and achieve the effects of high precision, strong practicability, and high purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
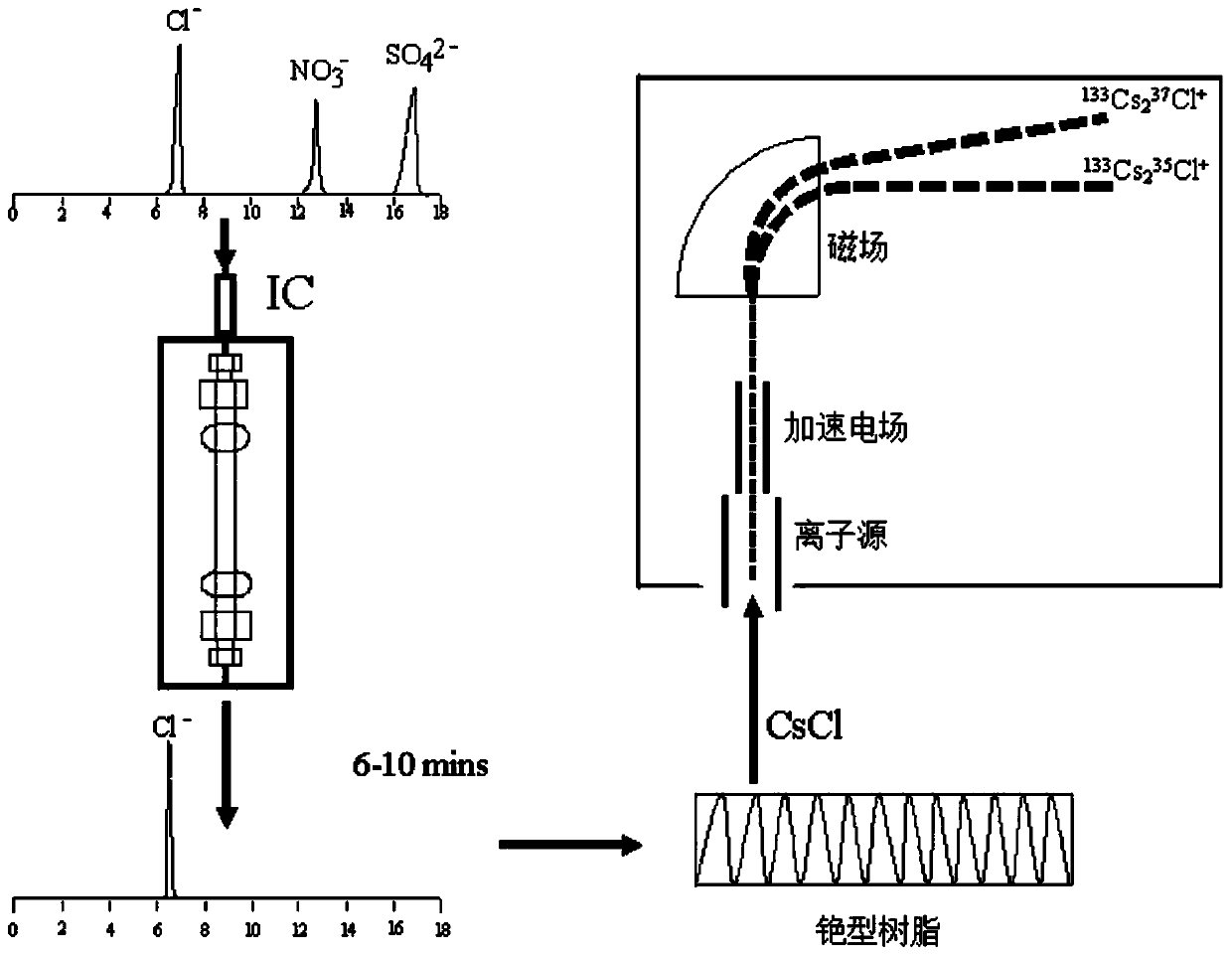
[0036] To measure the ratio of chlorine isotopes in groundwater, river water and rainwater, the steps are as follows:
[0037] A. Removal of organic matter in samples and purification of samples:
[0038] Take a reversed-phase solid-phase extraction column (C18, 1000mg / 6.0mlENVI-18, produced by SUPELCO Company), sequentially use 2mL methanol and 10mL pure water to activate the solid-phase extraction column, and then pass the sample solution through the activated reverse-phase Solid phase extraction cartridge, the flow rate is controlled at 5mL / min;
[0039] B. Removal and separation and purification of inorganic interfering substances in samples:
[0040] B-1: Setting of Ion Chromatography Conditions
[0041] Debug the signal trigger device and fraction collector of the isotope-specific preparative ion chromatograph (developed by the Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences, model: Pre-Isotope) to ensure that the instrument ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com