Preparation method and applications of alkylimidazole type ion liquid functionalized quinine silica gel chromatography stationary phase
An alkylimidazole-type, ionic liquid technology, applied in the field of chromatographic stationary phase, to achieve the effect of easy-to-obtain raw materials in the preparation process, novel stationary phase structure, and realize the effect of commercial batch production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
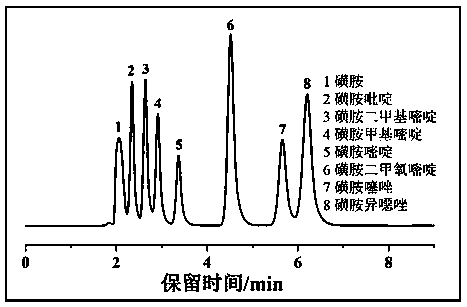
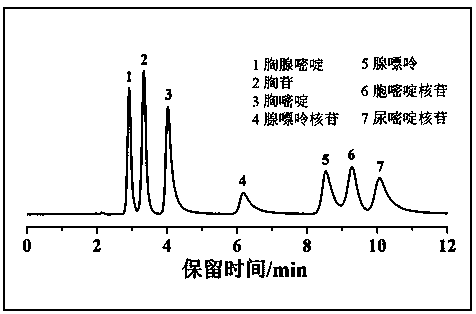
Examples
Embodiment 1
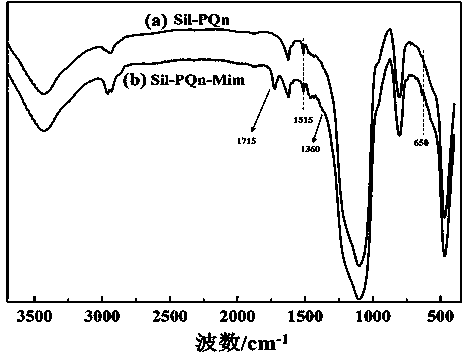
[0061] (1) Ultrasonically disperse 3.0 g of 3-mercaptopropyltriethoxysilane coupling agent and 3.0 g of silica gel in 30.0 ml of dimethylbenzene solvent, and raise the temperature to 90°C under the protection of nitrogen or argon for mechanical stirring reaction After 48 h, cool to room temperature, wash by centrifugation, and dry in vacuum to obtain mercaptopropyl silica gel;
[0062] (2) Ultrasonic disperse 2.8 g of mercaptopropyl silica gel, 2.0 g of quinine, and 28.0 mg of azobisisobutyronitrile in 20.0 ml of anhydrous chloroform (add molecular sieve to remove water), heat up to 65°C and mechanically stir and reflux the reaction After 30 hours, after the mixture was cooled to room temperature, it was centrifuged and washed, and dried in vacuum to obtain polyquinine silica gel;
[0063] (3) Add 2.1 ml of 2-chloroethyl isocyanate dropwise to 31.0 ml of anhydrous dichloromethane homogeneous dispersion containing 2.6 g of polyquinine silica gel, mechanically stir at room tempe...
Embodiment 2
[0067] (1) Ultrasonically disperse 2.5 g of 3-mercaptopropyltrimethoxysilane coupling agent and 3.0 g of silica gel in 42.0 ml of benzene solvent, raise the temperature to 100°C under the protection of nitrogen or argon atmosphere, and react with mechanical stirring for 20 h. Cool to room temperature, wash by centrifugation, and dry in vacuum to obtain mercaptopropyl silica gel;
[0068] (2) Ultrasonic disperse 2.8 g of mercaptopropyl silica gel, 2.8 g of quinine and 50.0 mg of azobisisobutyronitrile in 22.0 ml of anhydrous chloroform (add molecular sieve to remove water), heat up to 100°C and mechanically stir and reflux the reaction After 22 h, the mixture was cooled to room temperature, washed by centrifugation, and dried in vacuum to obtain polyquinine silica gel;
[0069] (3) Add 1.5 ml of chloroethyl isocyanate dropwise to 20.0 ml of anhydrous chloroform homogeneous dispersion containing 2.6 g of polyquinine silica gel, and react with mechanical stirring at room temperat...
Embodiment 3
[0073] (1) Ultrasonically disperse 3.5 g of 3-mercaptopropyltriethoxysilane coupling agent and 3.0 g of silica gel in 28.0 ml of toluene solvent, raise the temperature to 90°C under the protection of nitrogen atmosphere, and react with mechanical stirring for 36 h, then cool to room temperature, centrifugal washing, and vacuum drying to obtain mercaptopropyl silica gel;
[0074] (2) Ultrasonic disperse 2.8 g of mercaptopropyl silica gel, 2.5 g of quinine and 30.0 mg of azobisisobutyronitrile in 15.0 ml of anhydrous chloroform, heat up to 70°C and mechanically stir and reflux for 30 hours, and wait for the mixture to cool to After room temperature, centrifuge and wash, and vacuum dry to obtain polyquinine silica gel;
[0075] (3) Add 1.8 ml of 3-chloropropyl isocyanate dropwise to 25.0 ml of anhydrous chloroform homogeneous dispersion containing 2.6 g of polyquinine silica gel, mechanically stir at room temperature and react for 22 h, centrifuge, wash, and vacuum dry. Obtain p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com