Co-Mn catalyst for catalytic oxidation of NH3, preparation method and applications thereof
A catalytic oxidation and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of low nitrogen selectivity, poor selectivity, and poor improvement effect. Obvious and other problems, to achieve the effect of high N2 selectivity, low cost, and excellent N2 selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] WO 3 -CoMn 2 o 4 Catalyst preparation:
[0031] (1) 50wt% manganese nitrate solution is dispersed in ethanol-water solution (wherein ethanol is 1ml, water is 10ml), stirs at 25 ℃, forms the manganese nitrate solution of 10ml0.2mol / l. Slowly add 10ml of 25wt% concentrated ammonia solution dropwise, and keep stirring for 1h. After fully mixing and reacting, record it as solution A. Dissolve and disperse cobalt nitrate in ethanol-water solution (1ml of ethanol and 10ml of water), stir at 25°C to form 10ml of 0.1mol / l cobalt nitrate solution, which is referred to as solution B. The solution B was slowly added dropwise into the solution A, and the stirring time was continued at 25° C. for 1 h, and the reaction was fully mixed. Then transferred to constant temperature heating equipment, reacted at 180°C for 3h, cooled, and calcined at 200°C in an air atmosphere for 5h, with a heating rate of 2°C / min to obtain spinel-structured CoMn 2 o 4 , remember Catalyst A.
[0032]...
Embodiment 2
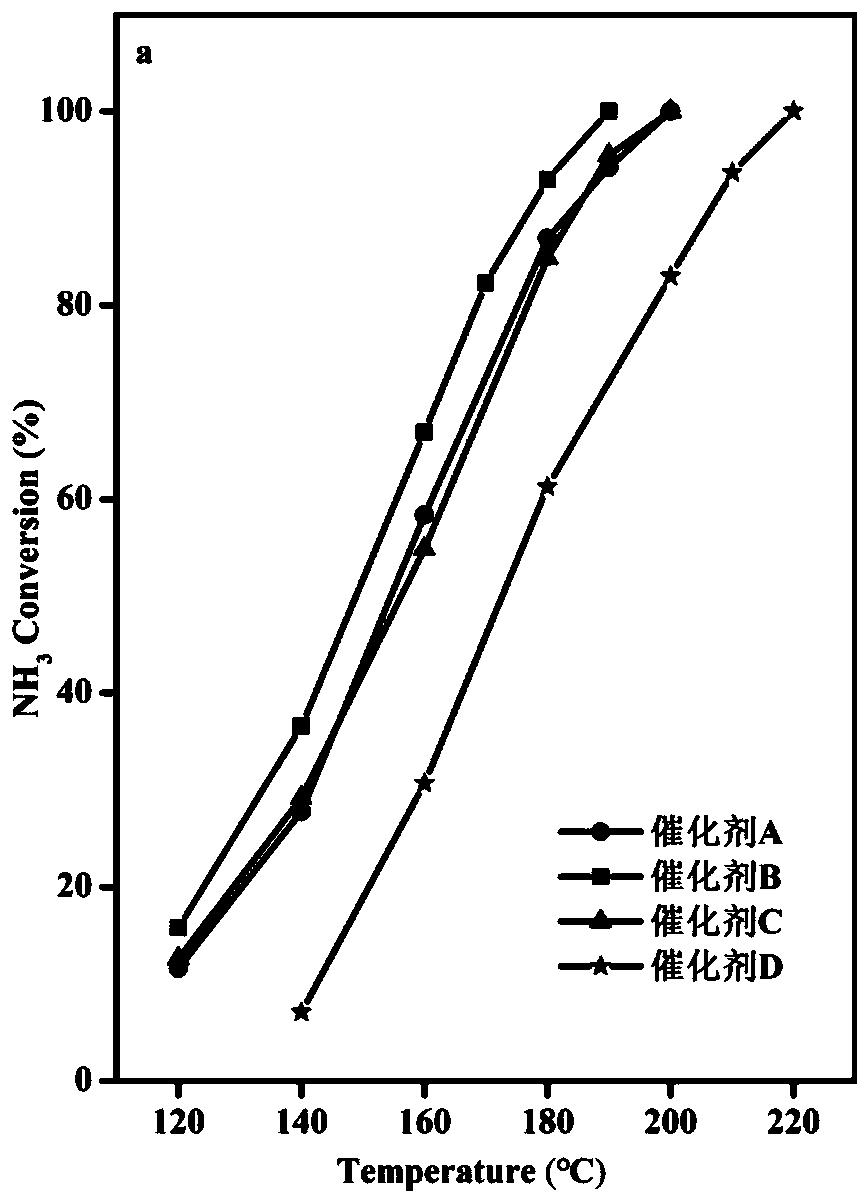
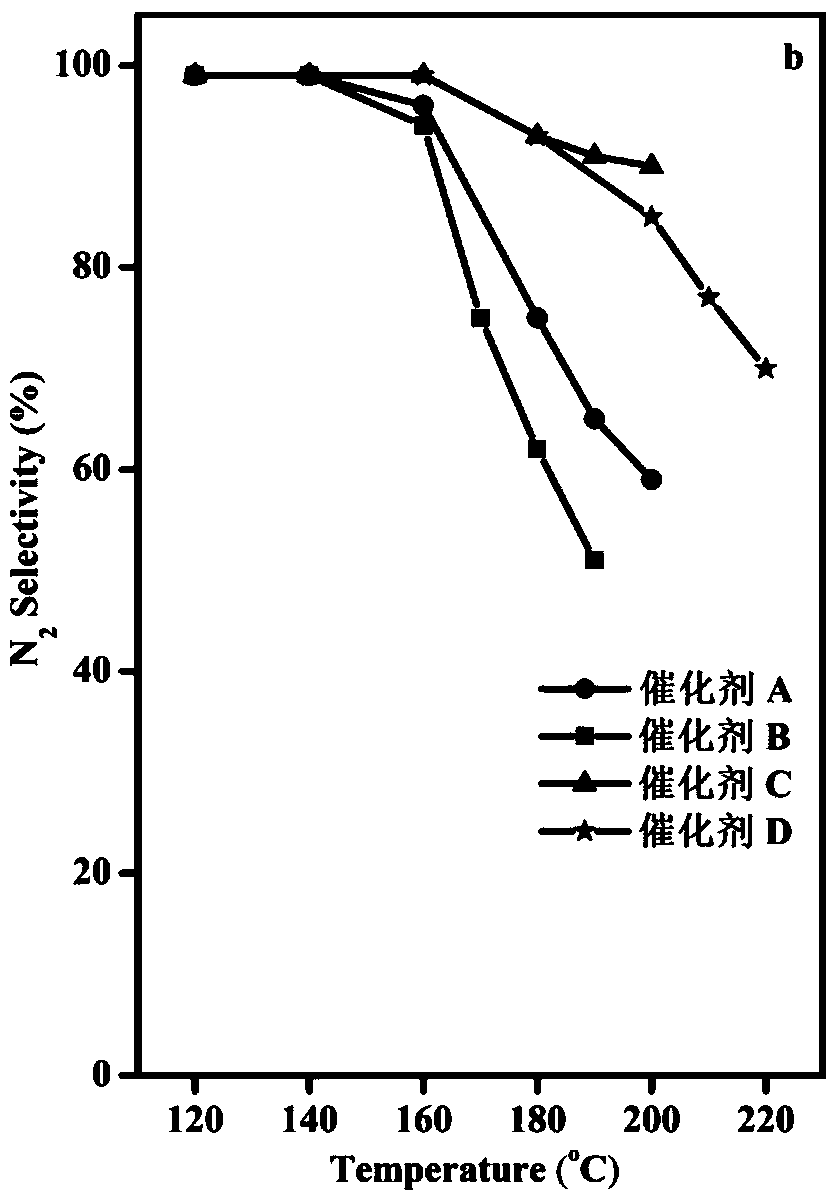
[0043] The WO prepared in Example 1 3 -CoMn 2 o 4 Catalyst and the Co-Mn (CoMn 2 o 4 ), Co-Mn soaked by tetrabutylammonium hydroxide (CoMn 2 o 4 ) and TiO 2 -CoMn 2 o 4 carry out NH 3 Catalytic oxidation, NH 3 Catalytic oxidation performance test is carried out in continuous operation on a fixed-bed reactor, He is used as balance gas, and NH 3 Analyzer and gas chromatograph on-line analyze the gaseous substance NH after the reaction 3 and N 2 .
[0044] The specific reaction conditions are: 1000ppm NH 3 , 10vol%O 2 , He is used as the balance gas, and the reaction space velocity is 40000h -1 , the mass of catalyst is 0.2g. NH 3 Conversion and N 2 The selectivity calculation formula is as follows: NH 3 Conversion %=[(NH before reaction 3 Concentration - NH after reaction 3 Concentration) / NH before reaction 3 Concentration]*100, N 2 Selectivity=[(N after reaction 2 Concentration - N before reaction 2 Concentration) / 500 / (NH before reaction 3 Concentratio...
Embodiment 3
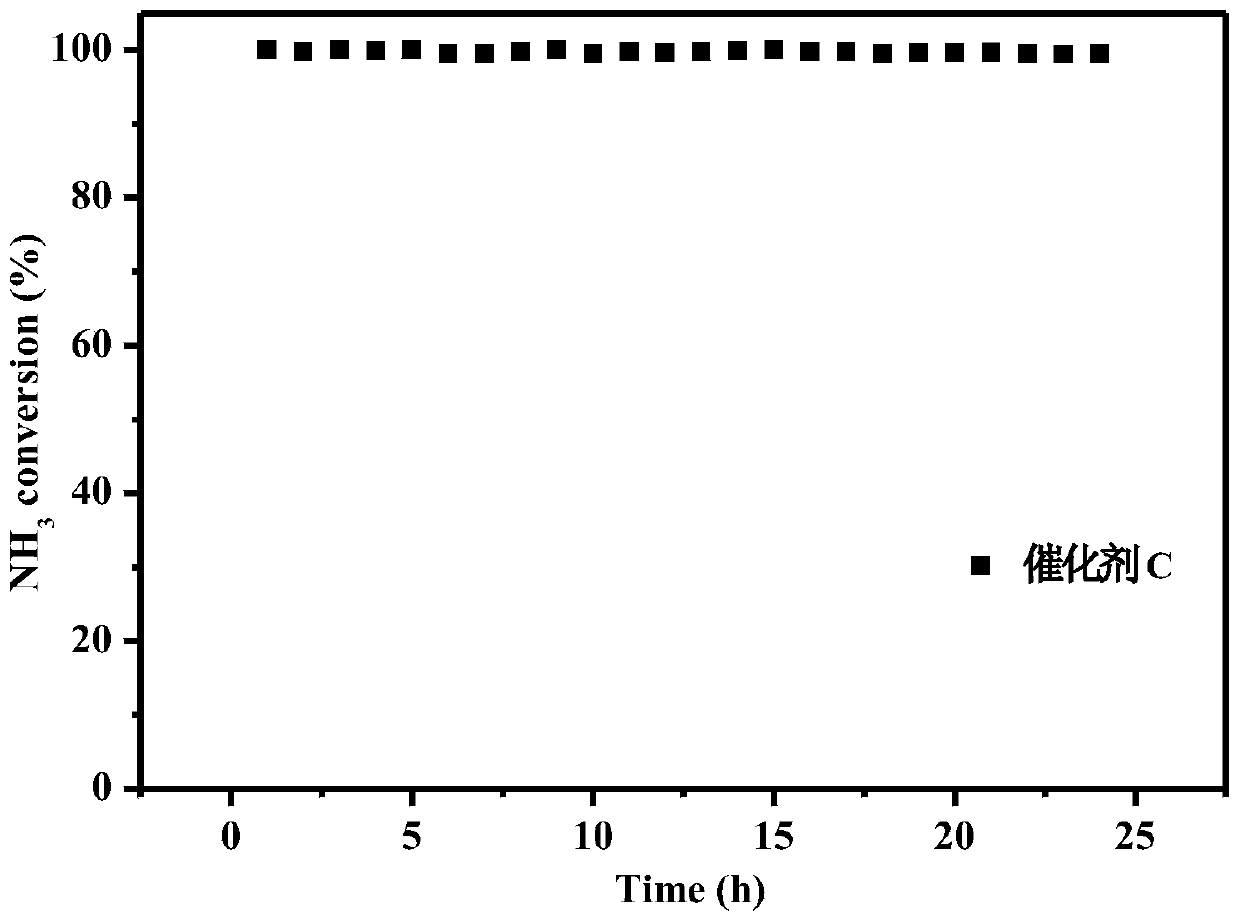
[0046] To the WO prepared in Example 1 3 -CoMn 2 o 4 Catalyst, investigate the stability of this catalyst, keep embodiment 2 reaction conditions, then in 200 ℃ of continuous operation tests the stability performance of catalyst, its stability performance is as figure 2 shown. After 24 hours of stable operation of the catalyst, the NH 3 Catalytic performance and N 2 The selectivity remains unchanged, showing excellent stability performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com