Rhizoma alismatis-largehead atractylodes rhizome tablet quality control method
A quality control method and technology of Zeshu tablets, applied in the field of medicine, can solve problems such as inability to meet quality control, and achieve the effects of ensuring safety and effectiveness, ensuring stability and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
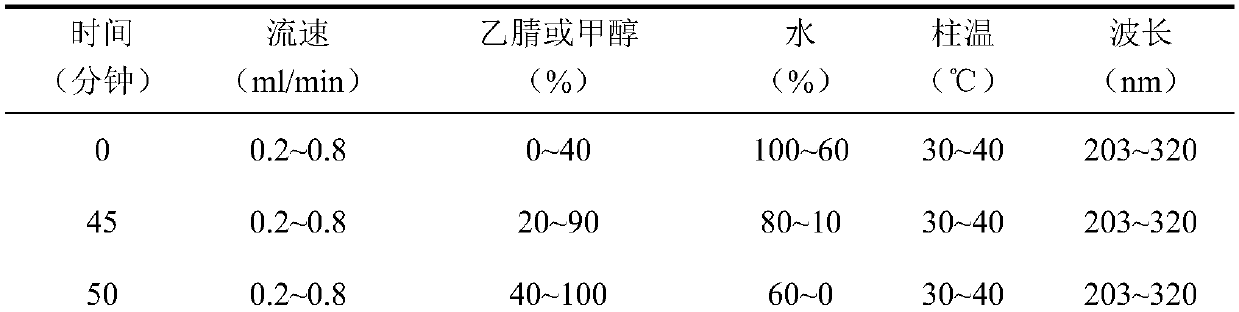
[0091] Chromatographic conditions and system suitability test: use C 18 Silica gel is used as a filler; the mobile phase is acetonitrile-water, and the gradient elution is carried out in the following table 10:
[0092] Table 10 gradient elution conditions
[0093]
[0094] Preparation of the test solution: Take 10 Zeshu Tablets, remove the coating layer, grind finely, accurately weigh, place in a stoppered Erlenmeyer flask, accurately add 25 mL of methanol, seal tightly, weigh, ultrasonically treat for 15 minutes, Allow to cool, weigh again, make up for the lost weight with methanol, shake well and let stand, take the supernatant through a 0.45 μm microporous membrane, and take the subsequent filtrate.
[0095]Preparation of reference substance solution: Accurately weigh the appropriate amount of 23-acetyl alisitol B reference substance, Atractylodes lactone Ⅰ, and Atractylodes lactone Ⅱ reference substance, and add methanol to make each 1ml contain 40.0 μg of 23-acetyl a...
Embodiment 2
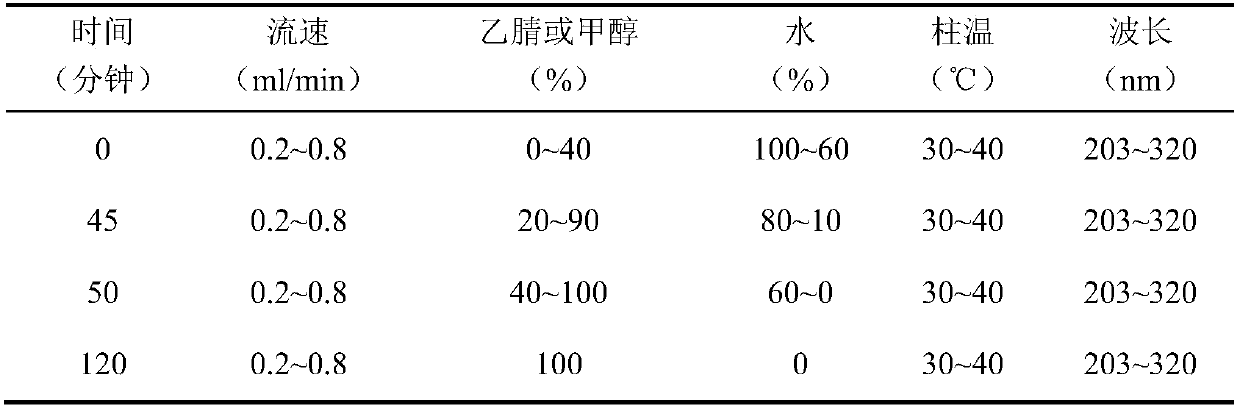
[0098] Chromatographic conditions and system suitability test: use C 8 Silica gel is used as a filler; the mobile phase is acetonitrile-0.05% acetic acid, and the gradient elution is carried out according to the following table 11:
[0099] Table 11 gradient elution conditions
[0100]
[0101] Preparation of the test solution: take 10 Zeshu tablets, remove the coating layer, grind finely, accurately weigh, place in a stoppered Erlenmeyer flask, accurately add 25mL of 50% methanol, seal tightly, weigh, and ultrasonically treat for 30 Minutes, let cool, weighed again, supplemented with methanol to reduce the lost weight, shake well and let it stand still, take the supernatant to pass through a 0.45μm microporous membrane, and take the subsequent filtrate.
[0102] Preparation of reference substance solution: Accurately weigh the appropriate amount of 23-acetyl alisitol B reference substance, Atractylodes lactone Ⅰ, and Atractylodes lactone Ⅱ reference substance, and add met...
Embodiment 3
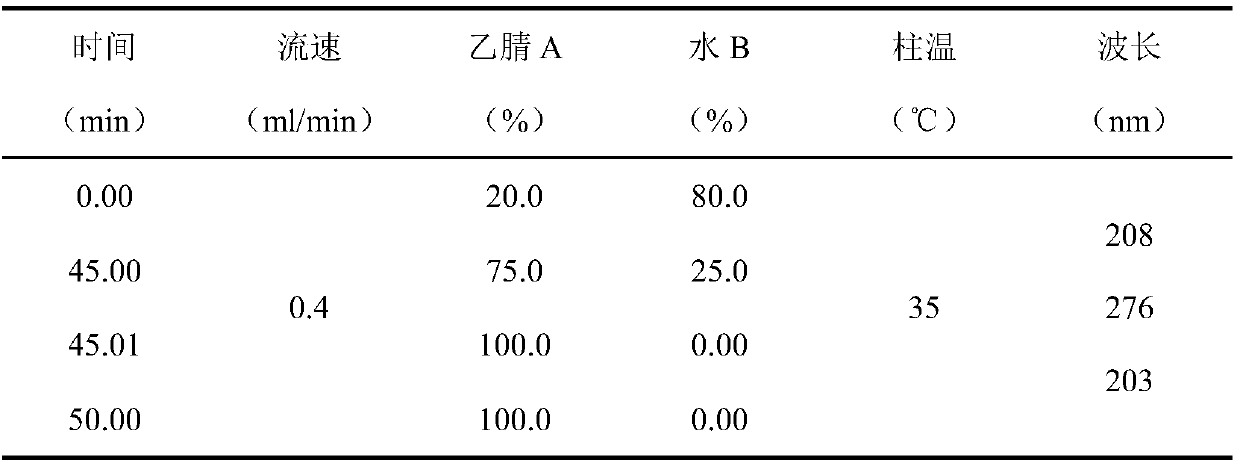
[0105] Chromatographic conditions and system suitability test: use C 8 Silica gel is used as a filler; the mobile phase is acetonitrile-0.05% phosphoric acid; gradient elution is carried out in the following table 12:
[0106] Table 12 gradient elution conditions
[0107]
[0108]
[0109] Preparation of the test solution: Take 10 Zeshu Tablets, remove the coating layer, grind finely, accurately weigh, place in a stoppered Erlenmeyer flask, accurately add 25mL of methanol, seal tightly, weigh, ultrasonically treat for 30 minutes, Allow to cool, weigh again, make up for the lost weight with methanol, shake well and let stand, take the supernatant through a 0.45 μm microporous membrane, and take the subsequent filtrate.
[0110] Preparation of reference substance solution: Accurately weigh the appropriate amount of 23-acetyl alisitol B reference substance, Atractylodes lactone Ⅰ, and Atractylodes lactone Ⅱ reference substance, and add methanol to make each 1ml contain 40....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com