Non-noble metal nitrogen-doped MOF double-effect electrocatalyst and preparation method thereof
A non-precious metal, electrocatalyst technology, applied in the direction of circuits, electrical components, battery electrodes, etc., can solve the problems of active sites of catalysts and immature reaction mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
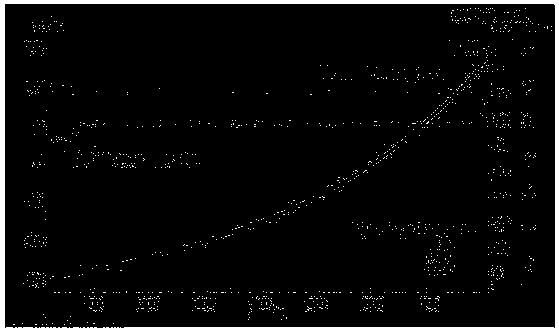
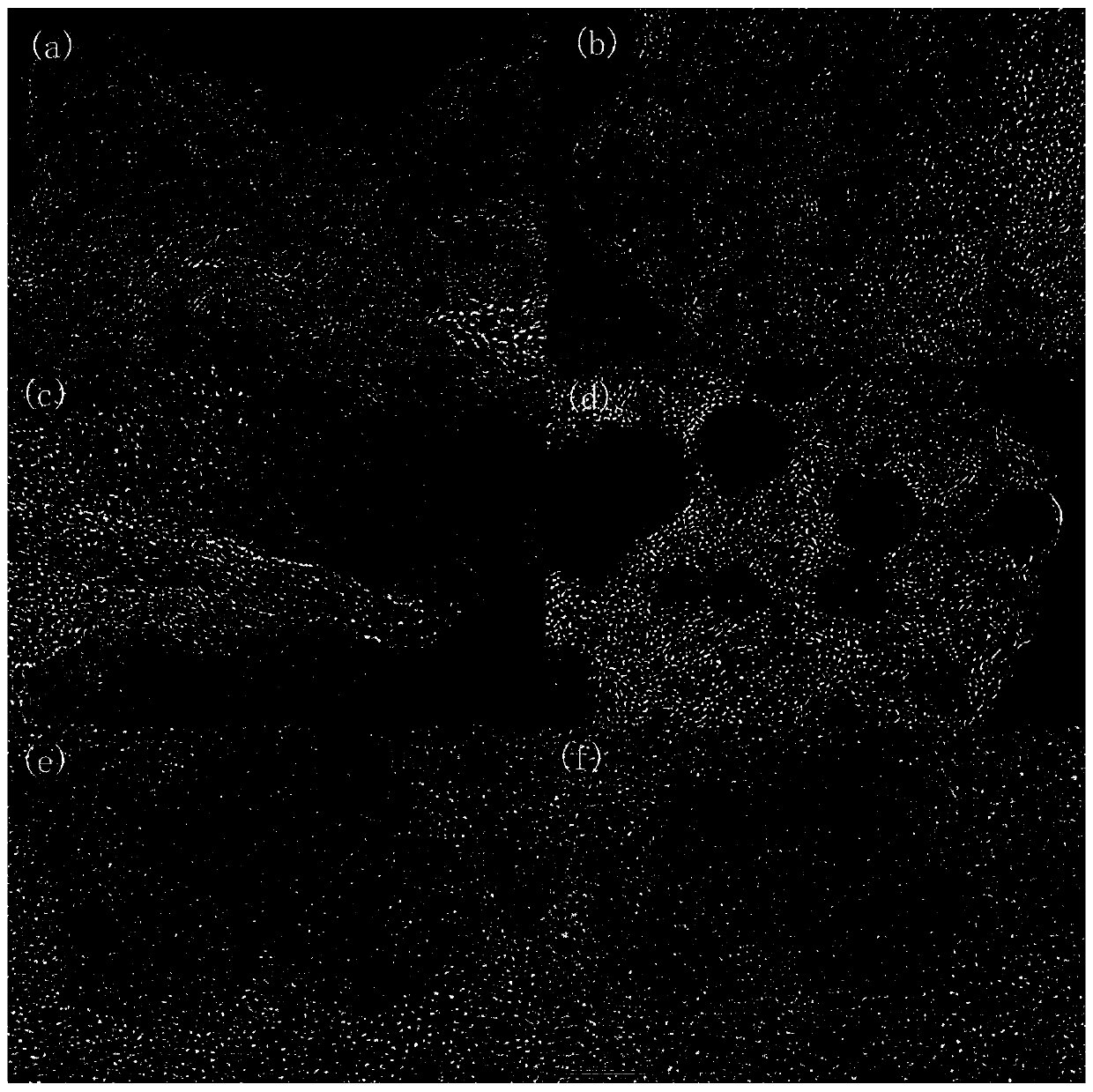
[0095] 0.5g of surfactant F127 was fully dissolved in 130mL of deionized H 2 Add 0.5g of tannic acid, 1.5g of imidazole and 1g of urea in sequence in O, and take an oil bath at 105°C to ensure that the organic matter of carbon and nitrogen sources is fully combined, and add 20mL of 0.1mol L -1 FeCl 3 Fully chelate with organic matter, and obtain the primary precursor at 105°C for 6h. Put the precursor in a high-pressure reactor at 105°C for 48 hours, and hydrothermally crystallize to ensure that the crystal grows fully, so as to obtain a uniformly dispersed precursor; the precursor is centrifugally dried and placed in N 2 Fully heat treatment at 5°C / min under the atmosphere, 800°C-2h, to obtain an active catalyst with a high degree of graphitization and a high specific surface area. Catalyst physical characterization as Figure 1-10 As shown, the catalyst performance is as Figure 11 , 12 , 13 shown.
Embodiment 2
[0097] 0.5g of surfactant F127 was fully dissolved in 130mL of deionized H 2 In O, add 2.8g of tannic acid, 2.8g of imidazole and 1g of urea in sequence, and take an oil bath at 105°C to ensure that the carbon and nitrogen sources are fully combined, and then add 20mL of 0.1mol L -1 FeCl 3 Fully chelate with organic matter, and obtain the primary precursor at 105°C for 6h. Put the precursor in a high-pressure reactor at 105°C for 48 hours, and hydrothermally crystallize to ensure that the crystal grows fully, so as to obtain a uniformly dispersed precursor; the precursor is centrifugally dried and placed in N 2 Fully heat treatment at 5°C / min under the atmosphere, 800°C-2h, to obtain an active catalyst with a high degree of graphitization and a high specific surface area. Catalyst performance comparison such as Figure 14 shown.
Embodiment 3
[0099] 0.5g of surfactant F127 was fully dissolved in 130mL of deionized H 2 In O, add 2.1g of tannic acid, 2.1g of imidazole and 1g of urea in sequence, and take an oil bath at 105°C to ensure that the carbon and nitrogen sources are fully combined, and then add 20mL of 0.1mol L -1 FeCl 3 Fully chelate with organic matter, and obtain the primary precursor at 105°C for 6h. Put the precursor in a high-pressure reactor at 105°C for 48 hours, and hydrothermally crystallize to ensure that the crystal grows fully, so as to obtain a uniformly dispersed precursor; the precursor is centrifugally dried and placed in N 2 Fully heat treatment at 5°C / min under the atmosphere, 800°C-2h, to obtain an active catalyst with a high degree of graphitization and a high specific surface area. Catalyst performance comparison such as Figure 14 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com