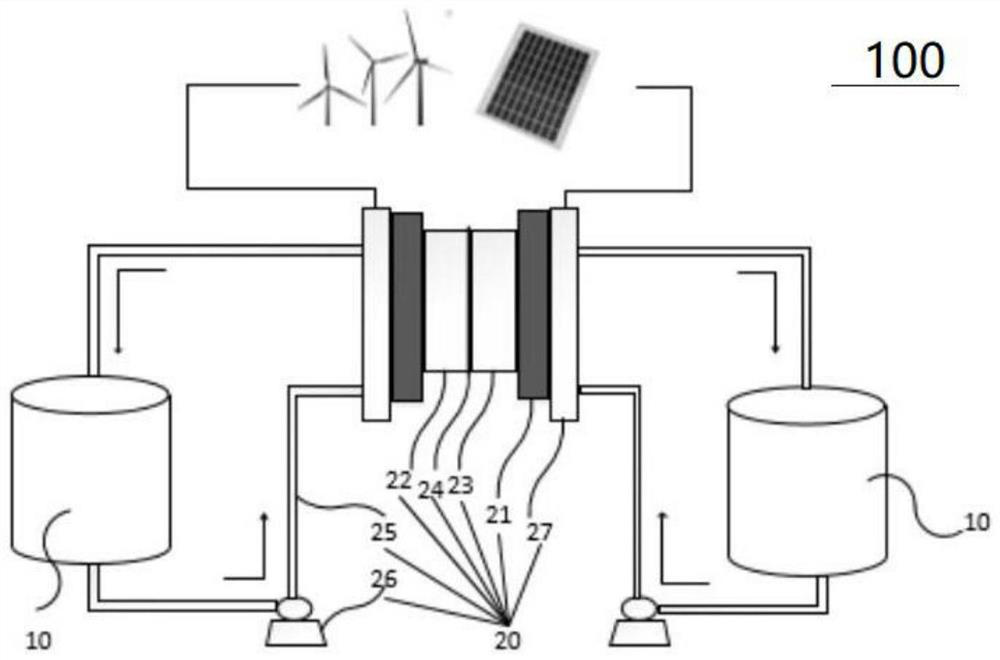
Neutral aqueous system liquid flow battery system
A technology of flow battery and flow battery stack, which is applied to fuel cells, regenerative fuel cells, circuits, etc., can solve the problems of prone to water electrolysis side reactions, limited solubility of active materials, and easy cross-contamination of electrolytes, and achieves cost-effectiveness. Low, high solubility, high safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
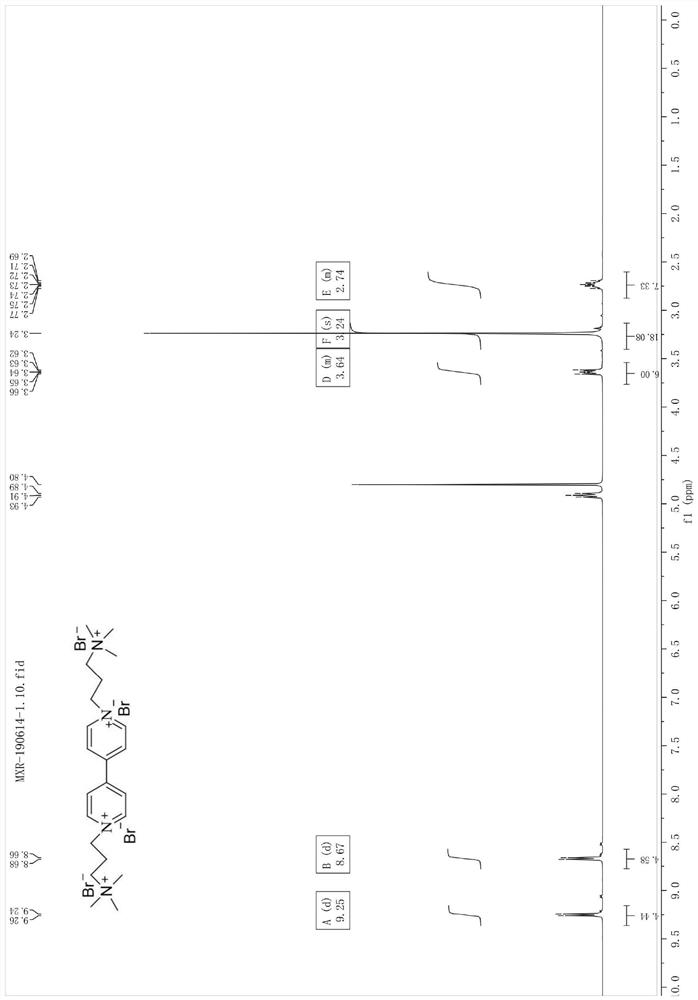
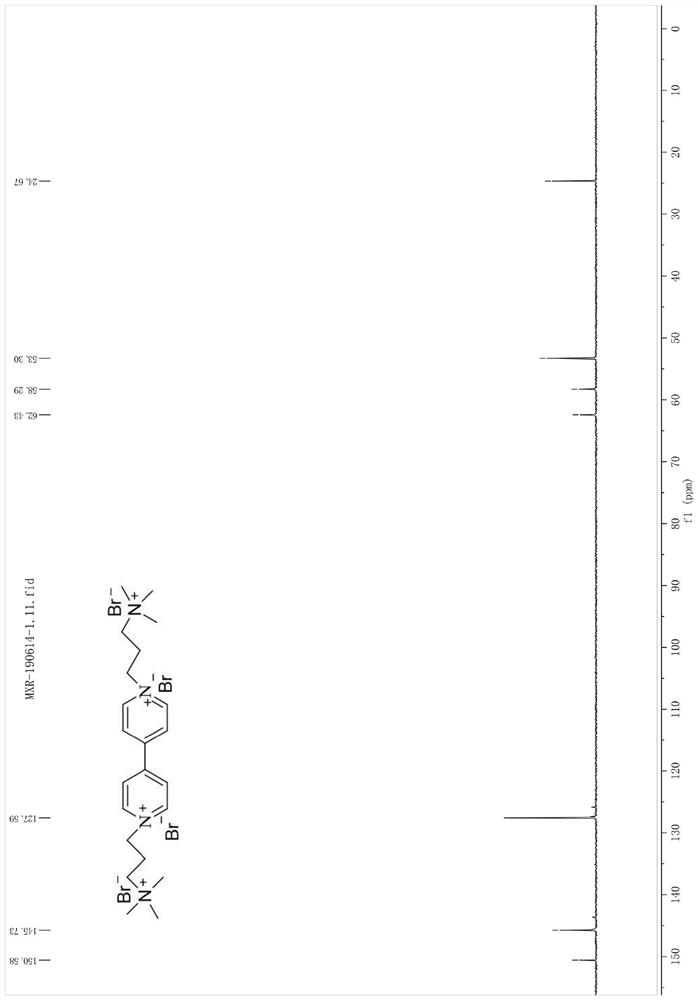
[0078] Synthesis of 1,1'-bis(3-(trimethylamino)propyl)-[4,4'-bipyridyl]tetrabromosalt
[0079] 0.47g (3.0mmol) of 4,4'-bipyridine and 1.27g (7.0mmol) of (3-bromopropyl)trimethylammonium bromide were mixed and dissolved in 30mL of acetonitrile. The temperature was raised slowly to reflux, and a light yellow solid was produced after 24 hours of reflux. Then the reaction solution was left standing overnight in the refrigerator. The precipitated product was filtered, washed and dried with ethanol, and recrystallized with methanol / ethanol (1:1, v / v) to obtain a solid. The product was characterized by NMR, and 1.43 g of the product was obtained with a yield of 92%.
[0080] Pale yellow solid, 1 HNMR (400MHz,D 2 O), δ9.25(d, J=7.0Hz, 4H), 8.67(d, J=6.9Hz, 4H), 5.04–4.85(m, 4H), 3.77–3.54(m, 4H), 3.24(s ,18H),2.87–2.60(m,4H); 13 C NMR (101MHz, D 2 O) δ 150.58, 145.73, 127.59, 62.43, 58.29, 53.30, 24.67.
Embodiment 2
[0082] Synthesis of (3-(trimethylamino)propyl)-[4,4'-bipyridyl]tribromide
[0083] 0.47g (3.0mmol) of 4,4'-bipyridine and 0.54g (3.0mmol) of (3-bromopropyl)trimethylammonium bromide were mixed and dissolved in 30mL of acetonitrile. The temperature was raised slowly to reflux, and a white solid was produced after reflux for 24 hours. Then the reaction solution was left standing overnight in the refrigerator. The precipitated product was filtered, washed and dried with ethanol, and recrystallized with methanol / ethanol (1:1, v / v) to obtain a solid. The product was characterized by NMR, and 0.91 g of the product was obtained with a yield of 90%.
[0084] white solid, 1 H NMR (400MHz,D 2 O)δ9.10(d, J=6.9Hz, 2H), 8.90–8.63(m, 2H), 8.48(d, J=6.9Hz, 2H), 8.04–7.80(m, 2H), 4.86(t, J=7.7Hz, 2H), 3.78–3.49(m, 2H), 3.25(s, 9H), 2.89–2.46(m, 2H); 13 C NMR (101MHz, D 2 O) δ154.32, 150.02, 144.99, 142.27, 126.45, 122.55, 62.50, 57.73, 53.35, 24.66.
Embodiment 3
[0086] Synthesis of 1,1'-bis(4-bromobutyl)-[4,4'-bipyridyl]dibromide
[0087] 0.47g (3.0mmol) of 4,4'-bipyridine and 1.51g (7.0mmol) of 1,4-dibromobutane were mixed and dissolved in 30mL of acetonitrile. The temperature was raised slowly to reflux, and a light yellow solid was produced after 24 hours of reflux. Then the reaction solution was left standing overnight in the refrigerator. The precipitated product was filtered, washed and dried with ethanol, and recrystallized with methanol / ethanol (1:1, v / v) to obtain a solid. The product was characterized by NMR, and 1.66 g of the product was obtained with a yield of 94.1%.
[0088] Pale yellow solid, 1 HNMR (400MHz,D 2 O), δ9.21(t, J=6.2Hz, 4H), 8.63(d, J=5.5Hz, 4H), 5.06–4.70(m, 7H), 3.60(t, J=6.4Hz, 2H), 2.52–2.20(m,4H),2.19–1.91(m,2H); 13 C NMR (101MHz,D 2 O) δ145.59, 127.38, 127.19, 61.33, 33.27, 29.41, 28.53.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com