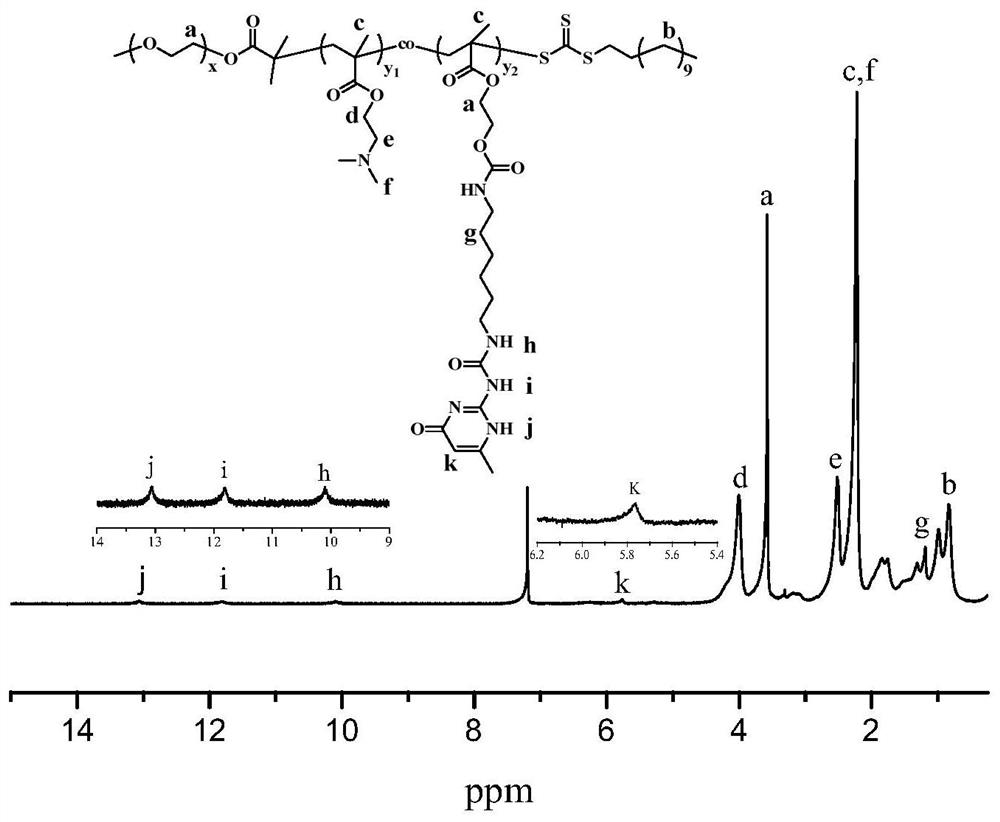
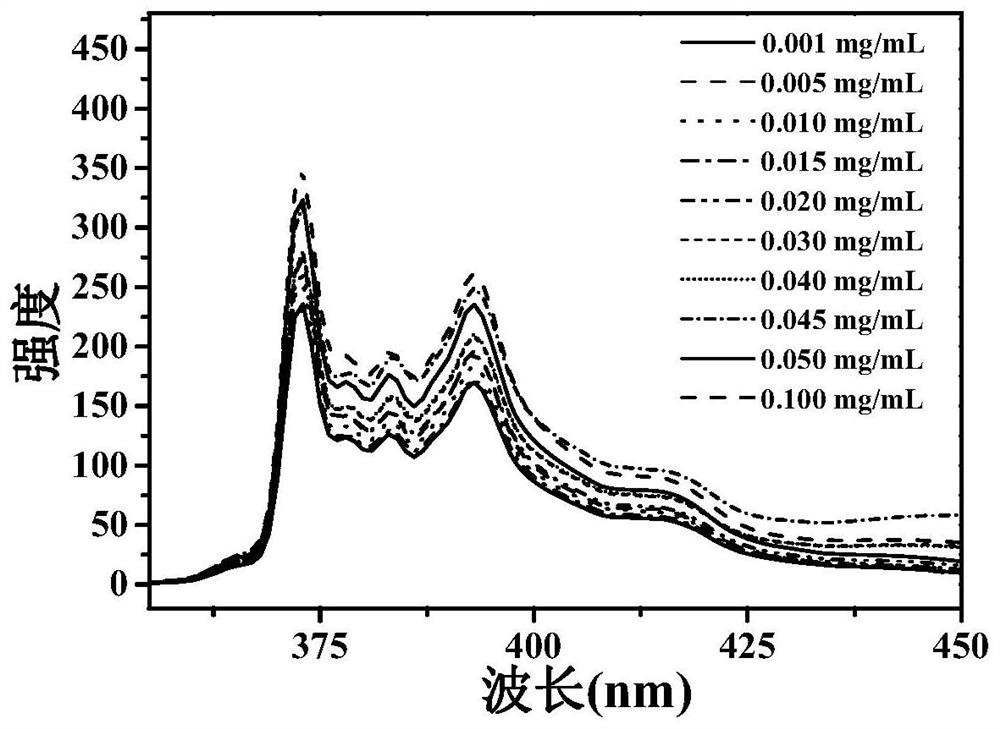
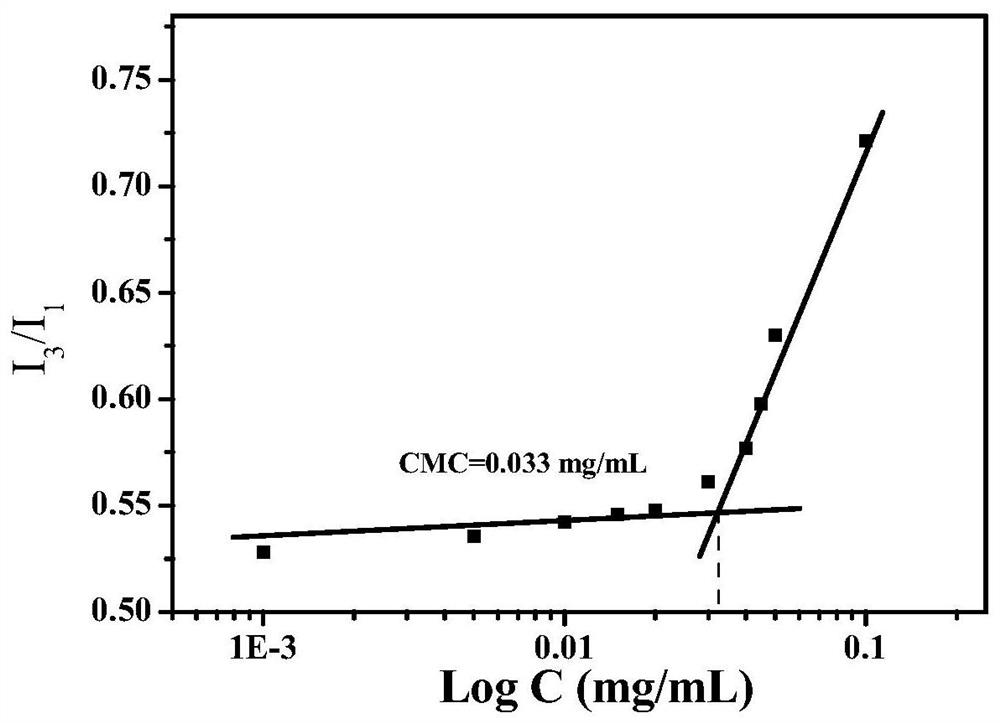
Responsive cross-linked polymer micelles based on multiple hydrogen bond interactions and their preparation methods and applications
A technology of cross-linked polymers and multiple hydrogen bonds, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, emulsion delivery, etc., can solve the problem of low targeting of cancer cells, premature release of drug delivery, and slow release speed And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] 1. Dissolve CTA (7.2800g, 20mmol), dry mPEG (10.0000g, 5mmol) and DMAP (0.2440g, 2mmol) in 60mL CH 2 Cl 2 After being completely dissolved, EDC (1.9170g, 10mmol) was added, reacted at 25°C for three days, and the insoluble matter was removed. After concentration, the solution was precipitated twice in ether to obtain a light yellow solid. The resulting solid was dissolved in 80 mL CH 2 Cl 2 In, with saturated Na 2 CO 3 The aqueous solution was washed three times, and the obtained organic phase was dried over anhydrous sodium sulfate for 12 hours. Filtration, after most of the solvent is removed by rotary evaporation, precipitation in excess glacial ether, to obtain light yellow powder solid, namely the macromolecular chain transfer agent (mPEG) shown in formula I-1 39 -CTA).
[0043] 2. The mPEG 39 -CTA (0.7002g, 0.3mmol), DMAEMA (1.5700g, 10mmol), UPy-HEMA (0.4367g, 1.1mmol) and AIBN (0.0164g, 0.1mmol) were added to a 50mL Shrek tube, and 5mL DMSO wa...
Embodiment 2
[0047]
[0048] 1. Dissolve CTA (6.552g, 18mmol), dry mPEG (4.0000g, 2mmol) and DMAP (0.2440g, 2mmol) in 60mL CH 2 Cl 2 After being completely dissolved, EDC (1.5336g, 8mmol) was added, and the insoluble matter was removed after three days of reaction at 25°C. The solution was concentrated and precipitated twice in ether to obtain a light yellow solid. The resulting solid was dissolved in 80 mL CH 2 Cl 2 In, with saturated Na 2 CO 3 The aqueous solution was washed three times, and the obtained organic phase was dried over anhydrous sodium sulfate for 12 hours. Filtrate, after rotary evaporation removes most of solvent, precipitate in excess glacial ether, obtain pale yellow powder solid, the macromolecular chain transfer agent (mPEG) shown in formula I-2 32 -CTA).
[0049] 2. The mPEG 32 -CTA (0.4668g, 0.2mmol), DMAEMA (1.4444g, 9.2mmol), UPy-HEMA (0.3176g, 0.8mmol) and AIBN (0.0164g, 0.1mmol) were added to a 50mL Shrek tube, and 5mL DMSO was added to make It was di...
Embodiment 3
[0053]
[0054] 1. Dissolve CTA (8.008g, 22mmol), dry mPEG (6.0000g, 3mmol) and DMAP (0.2440g, 2mmol) in 60mL CH 2 Cl 2 After being completely dissolved, EDC (2.3004g, 12mmol) was added, reacted at 25°C for three days, and the insoluble matter was removed. After concentration, the solution was precipitated twice in ether to obtain a light yellow solid. The resulting solid was dissolved in 80 mL CH 2 Cl 2 In, with saturated Na 2 CO 3 The aqueous solution was washed three times, and the obtained organic phase was dried with anhydrous sodium sulfate for 12 hours. Filtration, after rotary evaporation removes most of solvent, precipitate in excess glacial ether, obtain light yellow powder solid, the macromolecular chain transfer agent (mPEG) shown in formula I-3 35 -CTA).
[0055] 2. The mPEG 35 -CTA (0.2334g, 0.1mmol), DMAEMA (1.4915g, 9.5mmol), UPy-HEMA (0.1985g, 0.5mmol) and AIBN (0.0164g, 0.1mmol) were added to a 50mL Shrek tube, and 5mL DMSO was added to make It was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com