Application of fluorescent material based on fluorescent carbon dots in chemiluminiscence
A chemiluminescence and fluorescent carbon dot technology, applied to the application field of fluorescent materials in chemiluminescence, can solve the problems of complex preparation, high cost, poor compatibility, etc., and achieve the effects of simple detection equipment, low biological toxicity, and high application potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
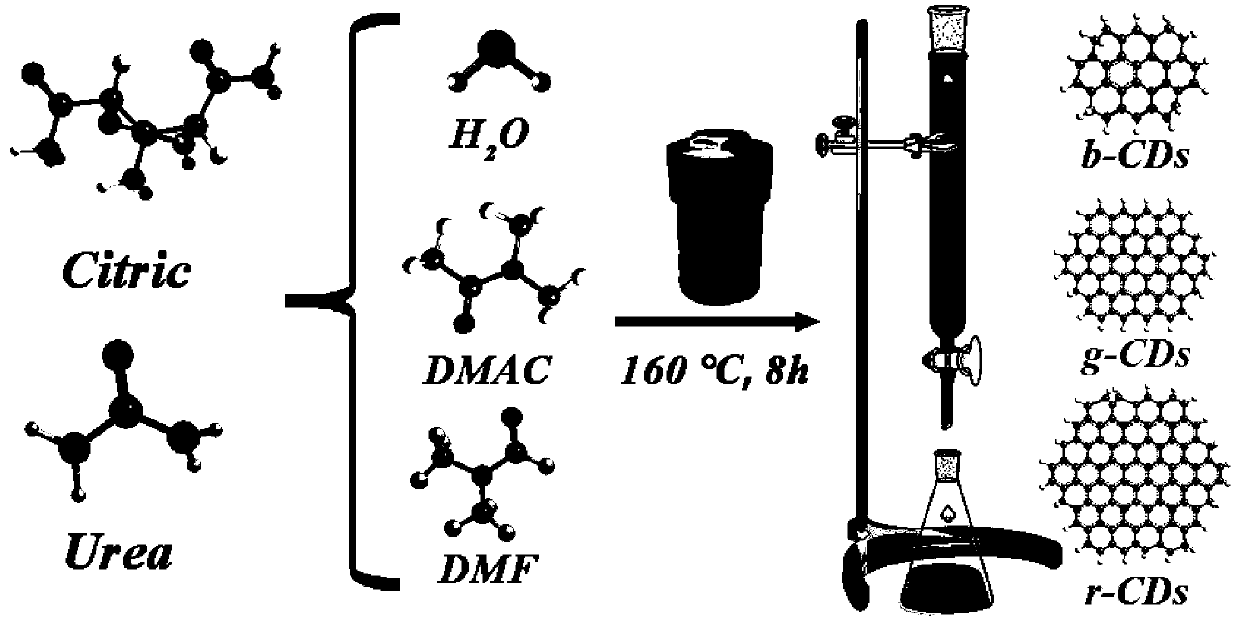
[0029] Embodiment 1. Preparation of fluorescent carbon dots by hydrothermal method
[0030] Step A. Weigh 1 g of citric acid with a mass fraction of 99% and 2 g of urea, add it to 10 mL of nitrogen-nitrogen dimethylformamide (DMF), and perform ultrasonic vibration at room temperature to obtain a mixed solution;
[0031] Step B. Place the shaken mixed solution in a high-temperature reaction furnace, heat it to 160° C., keep it warm for 8 hours, and then cool it down to room temperature naturally;
[0032] Step C. Dissolve the reacted mixed solution in DMF, use a silica gel chromatography column (200 mesh) to obtain a red fluorescent carbon dot (r-CDs) solution, add excess ethanol, filter, and retain the supernatant, Put it into a vacuum oven at 60°C for drying to obtain the required red fluorescent carbon dots.
[0033] The DMF in step A was replaced with nitrogen dimethylacetamide (DMAC), and the other steps remained unchanged to obtain the desired green fluorescent carbon do...
Embodiment 2
[0036] Embodiment 2. Preparation of fluorescent carbon dots by hydrothermal method
[0037] Step A. Weigh 1 g of citric acid with a mass fraction of 99% and 4 g of urea, add it to 10 mL of nitrogen-nitrogen dimethylformamide (DMF), and perform ultrasonic vibration at room temperature to obtain a mixed solution;
[0038] Step B. Place the shaken mixed solution in a high-temperature reaction furnace, heat it to 200°C, keep it warm for 4 hours, and then cool it down to room temperature naturally;
[0039] Step C. Dissolve the reacted mixed solution in DMF, use a silica gel chromatography column (200 mesh) to obtain a red fluorescent carbon dot (r-CDs) solution, add excess ethanol, filter, and retain the supernatant, Put it into a vacuum oven at 60°C for drying to obtain the required red fluorescent carbon dots.
[0040] The DMF in step A was replaced with nitrogen dimethylacetamide (DMAC), and the other steps remained unchanged to obtain the desired green fluorescent carbon dots...
Embodiment 3
[0042] Example 3. Preparation of fluorescent carbon dots by microwave method
[0043] Step A. Weigh 0.8g of citric acid and 1.6g of urea and dissolve them in deionized water, and carry out ultrasonic vibration at room temperature to disperse evenly to obtain a mixed solution;
[0044] Step B. The mixed solution was placed in a microwave oven, and after microwave heating for 5 minutes, the solution changed from colorless to brown solid;
[0045] Step C. Dissolve the reacted solid in excess ethanol, and after ultrasonic vibration, repeat the centrifugation three times, keep the supernatant, and dry it in a vacuum oven at 60°C to obtain the required fluorescent carbon dots.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com