Preparation technology of radix salviae miltiorrhizae tablets
A preparation process, a technology of Danshen decoction pieces, applied to medical preparations with no active ingredients, medical preparations containing active ingredients, plant raw materials, etc., can solve the problem of unstable sources, large differences in the content of index components, and poor efficacy of taking medicines, etc. problems, to achieve the effect of stable content and yield, stable and controllable content, and neat appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] ※Preparation of reference substance solution: Accurately weigh an appropriate amount of salvianolic acid B reference substance, add methanol-water (8:2) mixed solution to make a solution containing 0.10mg per 1ml, and obtain it.
[0048] ※Preparation of the test solution: Take about 0.15g of the powder of this product (passed through a No. 3 sieve), weigh it accurately, put it in a stoppered Erlenmeyer flask, add 50ml of methanol-water (8:2) mixed solution, and seal it tightly. , weighed, ultrasonically treated (power 140W, frequency 42kHz) for 30 minutes, allowed to cool, weighed again, and made up the lost weight with methanol-water (8:2) mixed solution, shake well, filter, and accurately measure Take 5ml of the continued filtrate, transfer it to a 10ml measuring bottle, add methanol-water (8:2) mixed solution to dilute to the mark, shake well, filter, take the continued filtrate, that is.
[0049] ※Determination method: Precisely draw 10μl each of the reference subst...
Embodiment 1
[0052] Every 1000 coated tablets is made of the following raw materials and auxiliary materials in the weight ratio: 1500g of spray-dried salvia miltiorrhiza root powder, 33g of calcium sulfate, 20.5g of starch, 16g of talcum powder, and 1% of the dry granule amount of magnesium stearate.
[0053] Its production process is as follows:
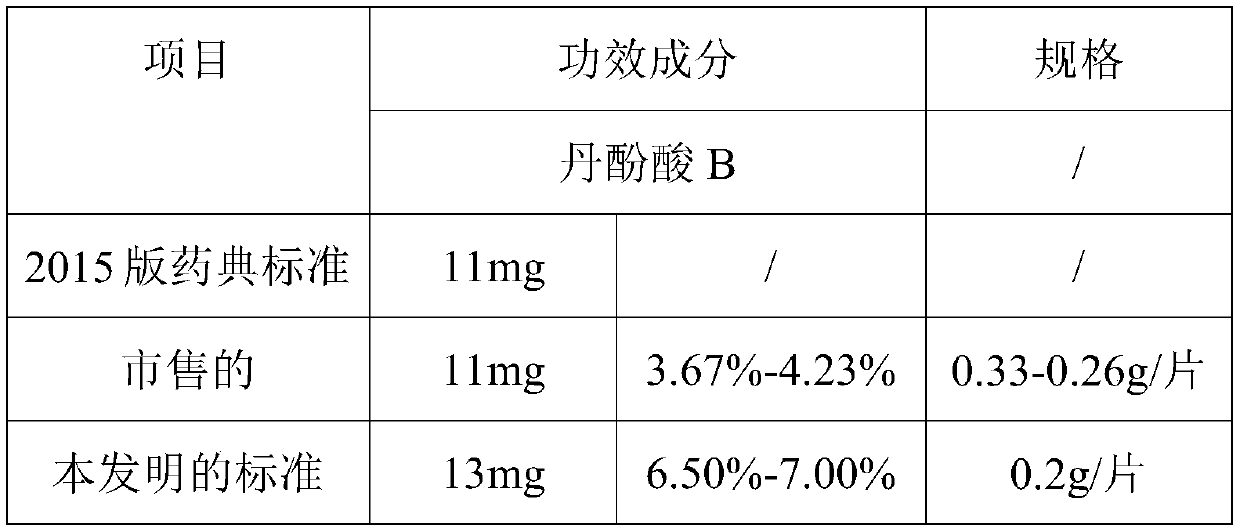
[0054] (1) Cut the two different medicinal materials of Salvia miltiorrhiza in total 1000g into inch segments, and detect the salvianolic acid B content and extract; the salvianolic acid B content is 6.5%; 8.8%; the extract is 82.9% and 64.5%.
[0055] (2) According to the test results, two different decoction pieces were prepared in proportion to extract 70.0% and salvianolic acid B 8.1%;
[0056] (6) Put the formula medicinal material in the extraction tank, add 3 times the volume of 90% ethanol to extract for 1.0 hour, filter, and concentrate the alcohol extract for later use;
[0057] (4) Add 3.5 times the volume of water to the dregs, ext...
Embodiment 2
[0063] Every 1000 coated tablets is made of the following raw materials and auxiliary materials in the weight ratio: 1500g of spray-dried salvia miltiorrhiza root powder, 33g of calcium sulfate, 20.5g of starch, 16g of talcum powder, and 1% of the dry granule amount of magnesium stearate.
[0064] Its production process is as follows:
[0065] (1) Cut the two different medicinal materials of Salvia miltiorrhiza in total 1000g into inch segments, and detect the salvianolic acid B content and extract; the salvianolic acid B content is 6.5%; 8.8%; the extract is 82.9% and 64.5%.
[0066] (2) According to the test results, two different decoction pieces were prepared in proportion to extract 70.0% and salvianolic acid B 8.1%;
[0067] (6) Put the formula medicinal material in the extraction tank, add 3 times the volume of 90% ethanol to extract for 1.0 hour, filter, and concentrate the alcohol extract for later use;
[0068] (4) Add 3.5 times the volume of water to the dregs, ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com