Preparation method of soluble cellulose nasal dressing
A cellulose and completely dissolving technology, which is applied in the direction of pharmaceutical formulations, bandages, drug delivery, etc., can solve the problems of inability to support and fix nasal bone tissue, low mechanical strength of packing materials, and residual harmful substances, etc., and achieve good product conversion feasibility, The effect of good biosecurity and reducing safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
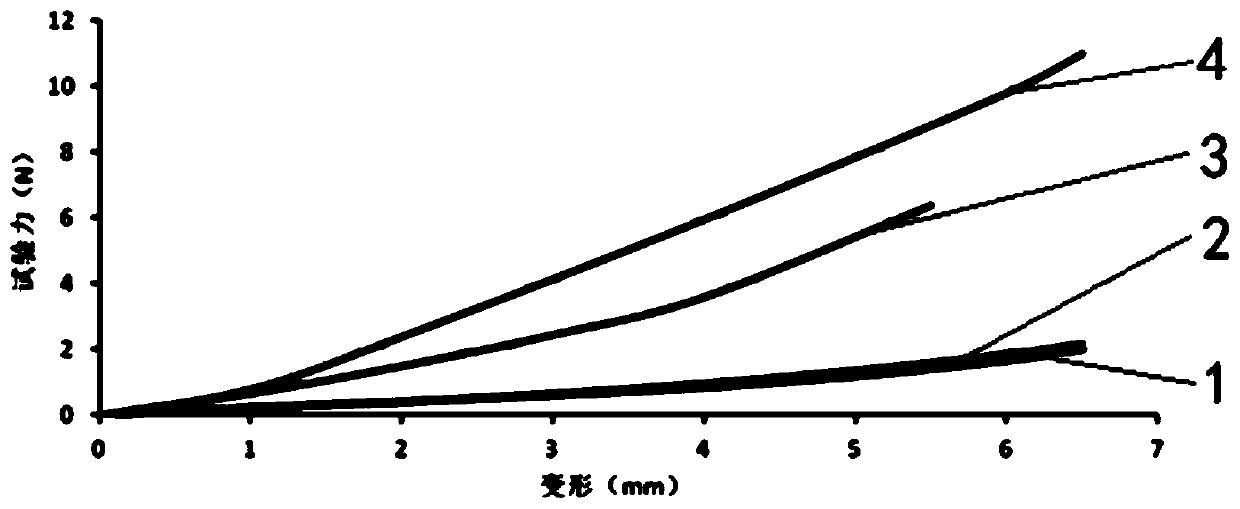
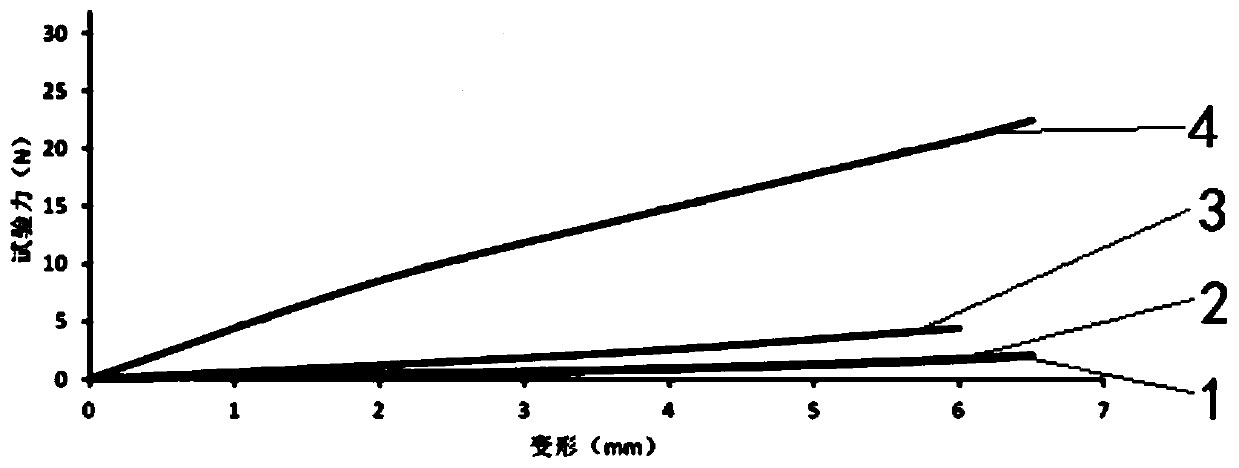
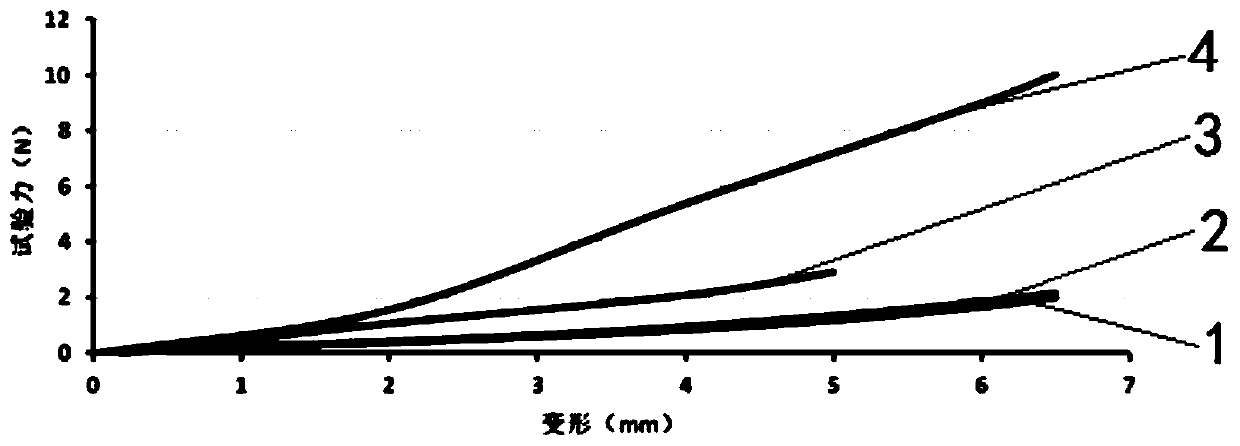
Image
Examples
Embodiment 1
[0027] A preparation method of dissolvable cellulose nasal dressing, comprising the following steps:
[0028] (1) Prepare an aqueous solution of hydrochloric acid with a certain mass concentration so that its pH is 4, slowly add a certain amount of CMC-Na powder to it, adjust the temperature to 25°C, stir at a speed of 100r / min to completely dissolve it, and form a 3wt % CMC-Na solution;
[0029] (2) Fill the CMC-Na solution gained in step (1) in a mould, put it into a freeze-drying box, set the initial temperature to 0°C, then gradually cool to -45°C to freeze, and then freeze the freeze-drying box Vacuum, and then raise the temperature to 40°C for drying to obtain a spongy solid;
[0030] (3) softening the spongy solid obtained in step (2) by freeze-drying at a temperature of 50° C. and a humidity of 50% for 48 hours;
[0031] (4) The nasal dressing softened in step (3) is sealed and packaged, and subjected to E-beam electron beam sterilization with a sterilization dose ra...
Embodiment 2
[0036] A preparation method of dissolvable cellulose nasal dressing, comprising the following steps:
[0037] (1) Prepare an aqueous solution of hydrochloric acid with a certain mass concentration so that its pH is 4, slowly add a certain amount of CMC-Na powder to it, adjust the temperature to 25°C, stir at a speed of 100r / min to completely dissolve it, and form 5wt % CMC-Na solution;
[0038] (2) Fill the CMC-Na solution gained in step (1) in a mould, put it into a freeze-drying box, set the initial temperature to 0°C, then gradually cool to -45°C to freeze, and then freeze the freeze-drying box Vacuum, and then raise the temperature to 40°C for drying to obtain a spongy solid;
[0039] (3) softening the spongy solid obtained in step (2) by freeze-drying at a temperature of 50° C. and a humidity of 50% for 48 hours;
[0040] (4) The nasal dressing softened in step (3) is sealed and packaged, and subjected to E-beam electron beam sterilization with a sterilization dose rang...
Embodiment 3
[0045] A preparation method of dissolvable cellulose nasal dressing, comprising the following steps:
[0046] (1) Prepare an aqueous solution of hydrochloric acid with a certain mass concentration so that its pH is 1, slowly add a certain amount of CMC-Na powder to it, adjust the temperature to 40°C, stir at a speed of 300r / min to completely dissolve it, and form a 3wt % CMC-Na solution;
[0047](2) Fill the CMC-Na solution gained in step (1) in a mould, put it into a freeze-drying box, set the initial temperature to 0°C, then gradually cool to -45°C to freeze, and then freeze the freeze-drying box Vacuum, and then raise the temperature to 40°C for drying to obtain a spongy solid;
[0048] (3) softening the spongy solid obtained in step (2) by freeze-drying at a temperature of 80° C. and a humidity of 90% for 15 hours;
[0049] (4) The nasal dressing softened in step (3) is sealed and packaged, and sterilized by gamma rays with a sterilization dose ranging from 25 to 35 KGy ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com