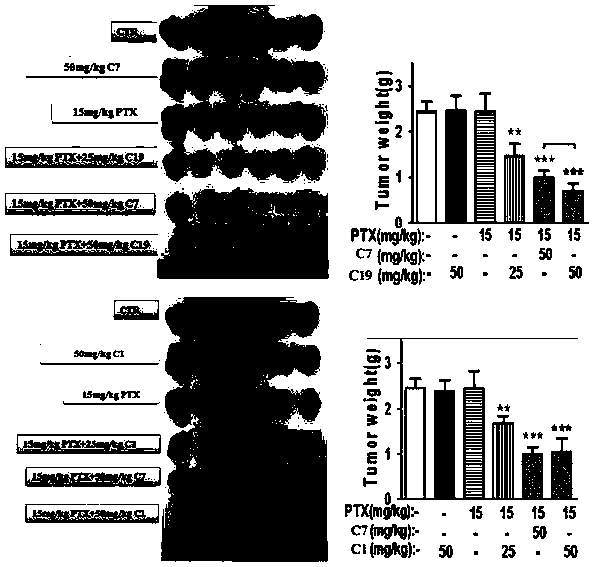
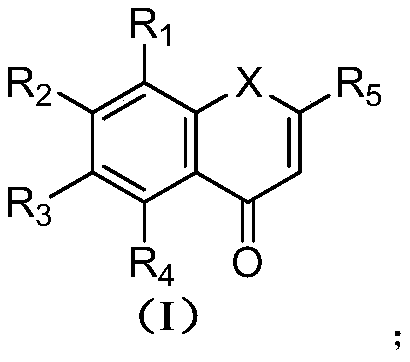
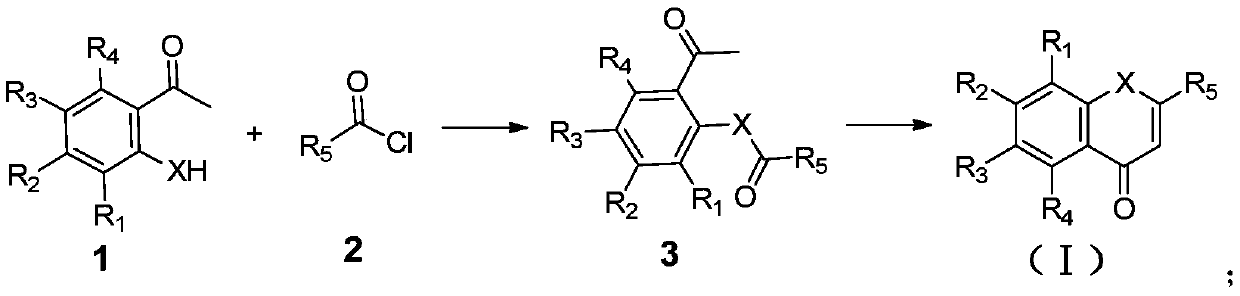
Nobiletin derivative or pharmaceutically acceptable salt thereof as well as preparation method and application thereof
A technology of nobiletin and derivatives, applied in organic chemistry, drug combination, antineoplastic drugs, etc., which can solve the problems of high toxicity and clinical use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] In the compound shown in formula (I), when X is O, its specific synthetic route is as follows:
[0077]
[0078] (1) Synthesis of Compound 12
[0079]
[0080] At room temperature, compound 3,4,5-trimethoxyphenol and sodium acetate were sequentially added to acetic anhydride and mixed, and heated at 110°C for 2 hours. After the reaction was completed, the reaction system was concentrated under reduced pressure and extracted three times with ethyl acetate. Washed with saturated brine and dried over anhydrous sodium sulfate. The organic layer was concentrated to obtain phenyl acetate containing different substituents, namely compound 12.
[0081] (2) Synthesis of Compound 13
[0082]
[0083] Compound 12 was added to glacial acetic acid solution, and boron trifluoride ether solution (about 48%) was added. The reaction mixture was stirred at 70°C for 2 hours and was monitored for completion by TLC analysis. Then the reaction system was quenched with water, ex...
Embodiment 2
[0097] The preparation of embodiment 2 compound C1
[0098] (1) Synthesis of compound 13a
[0099]
[0100] At room temperature, 3,4,5-trimethoxyphenol, compound 11a (9.2g, 50mmol) and sodium acetate (8.2g, 100mmol) were sequentially added to acetic anhydride (47mL, 500mmol) and mixed, heated at 110°C for 2 hours . After the reaction was completed, the reaction system was concentrated under reduced pressure and extracted three times with ethyl acetate. Washed with saturated brine and dried over anhydrous sodium sulfate. The organic layer was concentrated to obtain 3,4,5-trimethoxyphenethyl ester, namely compound 12a (11.2 g, 49.5 mmol). White solid, 99% yield. 1 H NMR (400MHz, CDCl 3 )δ6.34(s,2H), 3.83(s,9H), 2.29(s,3H).
[0101] Compound 12a (11.2 g, 49.5 mmol) was added to glacial acetic acid (37.5 mL), and boron trifluoride ether solution (48%, 250 mL) was added to the system. The reaction system was stirred at 70° C. for 4 hours, and the reaction effect was monit...
Embodiment 3
[0106] The preparation of embodiment 3 compound C2
[0107] (1) Synthesis of compound 13b
[0108]
[0109] The raw material is replaced by 2,3,4-trimethoxy-6-hydroxyacetophenone and nitric acid, and the product is obtained according to the method of step (2) in Example 2, that is, 2,3,4-trimethoxy 1-5-nitro-6-hydroxyacetophenone, yellow solid, yield: 79%. 1 H NMR (400MHz, CDCl 3 )δ13.56(s,1H), 4.10(s,3H), 4.07(s,3H), 3.81(s,3H), 2.69(s,3H).
[0110] (2) Synthesis of compound C2
[0111]
[0112] 5.0 mmol 2,3,4-trimethoxy-6-hydroxyacetophenone, 15 mmol triethylamine, 6.0 mmol 3,4-dimethoxybenzoyl chloride, dichloromethane (25 mL) solution. Obtain product 2-acetyl-3,4,5-trimethoxy-6-nitrophenyl-3,4-dimethoxy-methyl benzoate according to the method of step (4) in Example 1 , off-white solid, yield: 90%. 1 H NMR (500MHz, CDCl 3 )δ7.73(dd, J=8.5,2.0Hz,1H),7.52(d,J=2.0Hz,1H),6.92(d,J=8.5Hz,1H),4.06(s,3H),4.02( s,3H), 3.96(s,3H), 3.95(s,3H), 3.93(s,3H), 2.52(s,3H).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com