Alpha-glucosidase inhibitor, and synthesis method and application thereof
A technology of glucosidase and synthesis method, which is applied in the field of α-glucosidase inhibitor and its synthesis, and achieves the effects of good inhibitory activity and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of 1,2,4,5-tetramethoxybenzene (Intermediate A)
[0050]
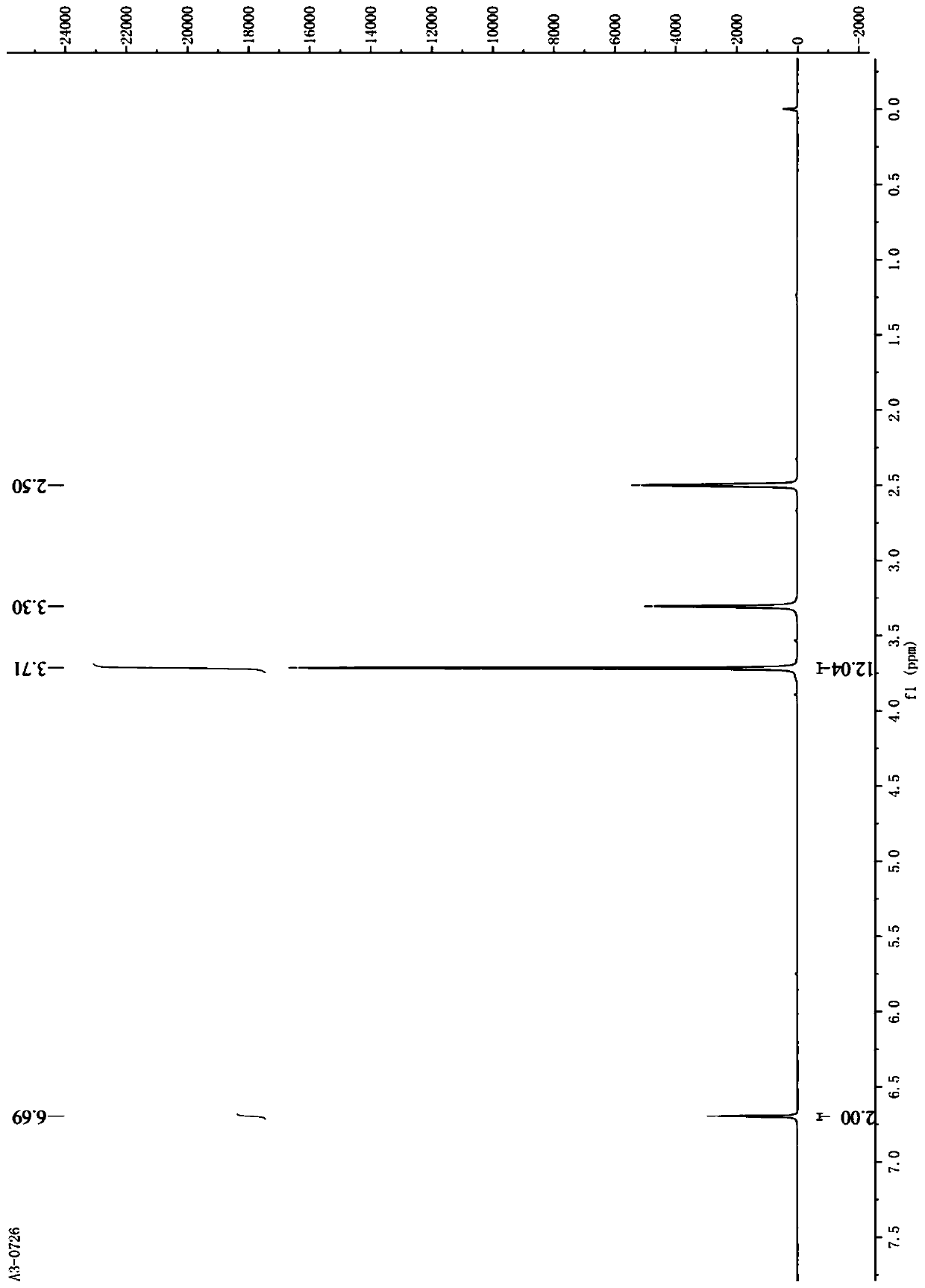
[0051]2,5 dihydroxy-1,4 benzoquinone (5 g, 3.6 mmol) was dissolved in 200 mL methanol solution, 38% concentrated hydrochloric acid (6 mL, 72.0 mmol) was added slowly, and stirred overnight at room temperature. After the reaction was completed (TLC), suction filtration and concentration gave a yellow solid intermediate product 1; the obtained intermediate product 1 was dissolved in 50 mL of water, sodium dithionite (10 g, 57.4 mmol) was added, and the reaction was refluxed for 8 minutes at 120 ° C. Cooling and crystallization at 4°C, the mixture was suction filtered to obtain powdery white crystalline intermediate 2 (4.5g, 73.5%); intermediate 2 (1.7g, 10mmol) was dissolved in 10mLDMSO, and potassium hydroxide (1.4g, 25mmol), stirred at room temperature for 15 minutes, added dropwise methyl iodide (1.88mL, 25mmol), and reacted overnight. The end point of the reaction was detected by TLC (developing ...
Embodiment 2
[0054] Synthesis of 1,2,4,5-tetramethoxy-3-(7-phenylheptyl)-benzene (intermediate B1)
[0055]
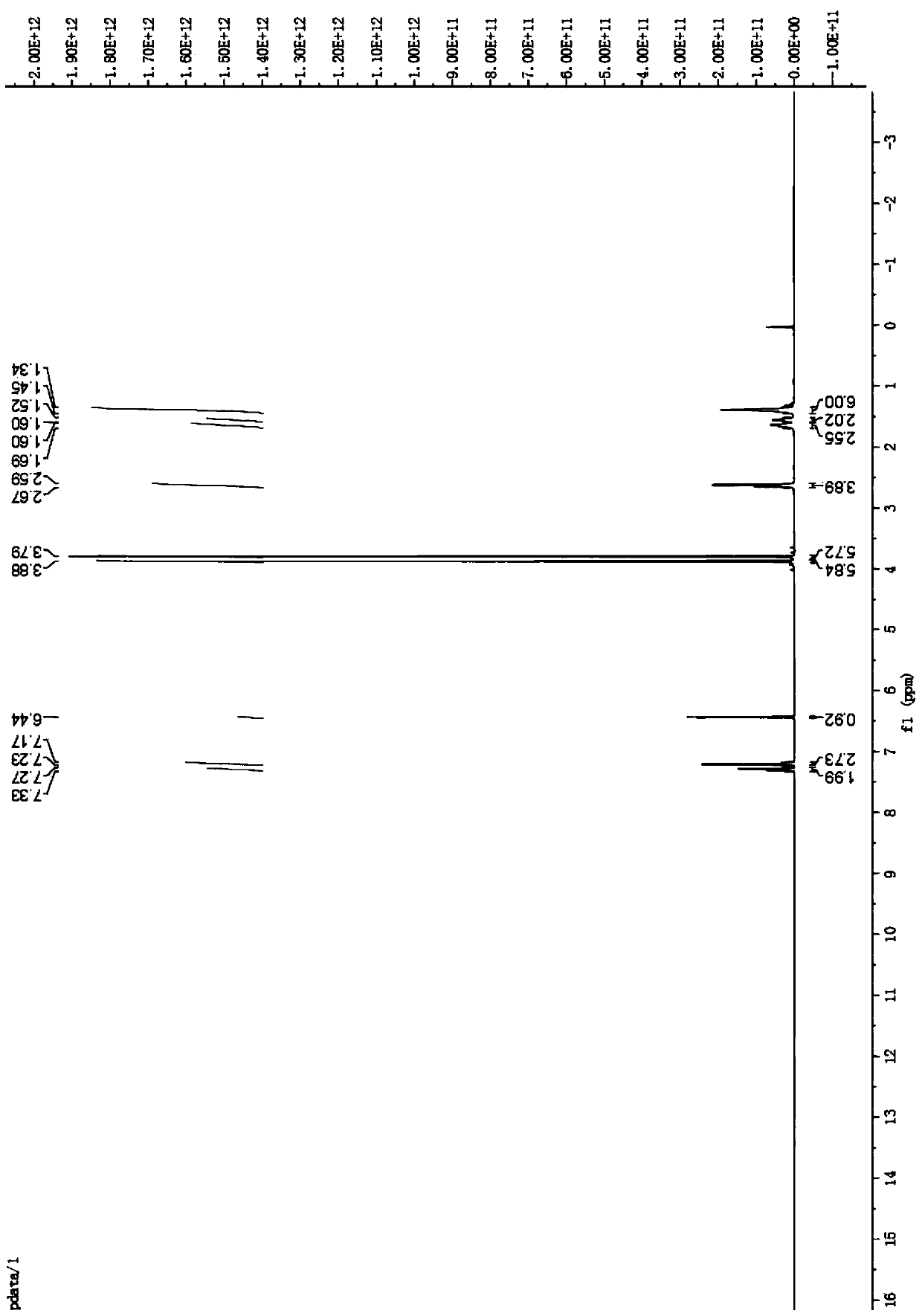
[0056] Add intermediate A (1 g, 5.1 mmol) into a round-bottomed flask, dry it in vacuum for 30 minutes, add HMPA (353 μL, 2 mmol), ventilate 2 to 5 times, and then add 60 mL of tetrahydrofuran as a solvent. React in anaerobic water for 15 minutes, slowly add n-butyllithium (2.44mL, 6.12mmol), adjust the temperature to -10°C for 1 hour, add 1-bromo-7-phenylheptane dropwise, and continue the reaction for 10 minutes . Then the mixture was reacted at room temperature for 10 hours. After concentrating the reaction liquid under reduced pressure, dissolve it with an organic solvent, adjust the pH to 5-6 with a saturated ammonium chloride solution, wash the organic phase with distilled water and saturated brine respectively, collect the organic phase and dry it with anhydrous magnesium sulfate, filter, and decompress Concentrate to obtain the crude product, which is purified by silica...
Embodiment 3
[0066] Synthesis of 1,2,4,5-tetramethoxy 3-(2-cyclohexylethyl)benzene (intermediate B2)
[0067]
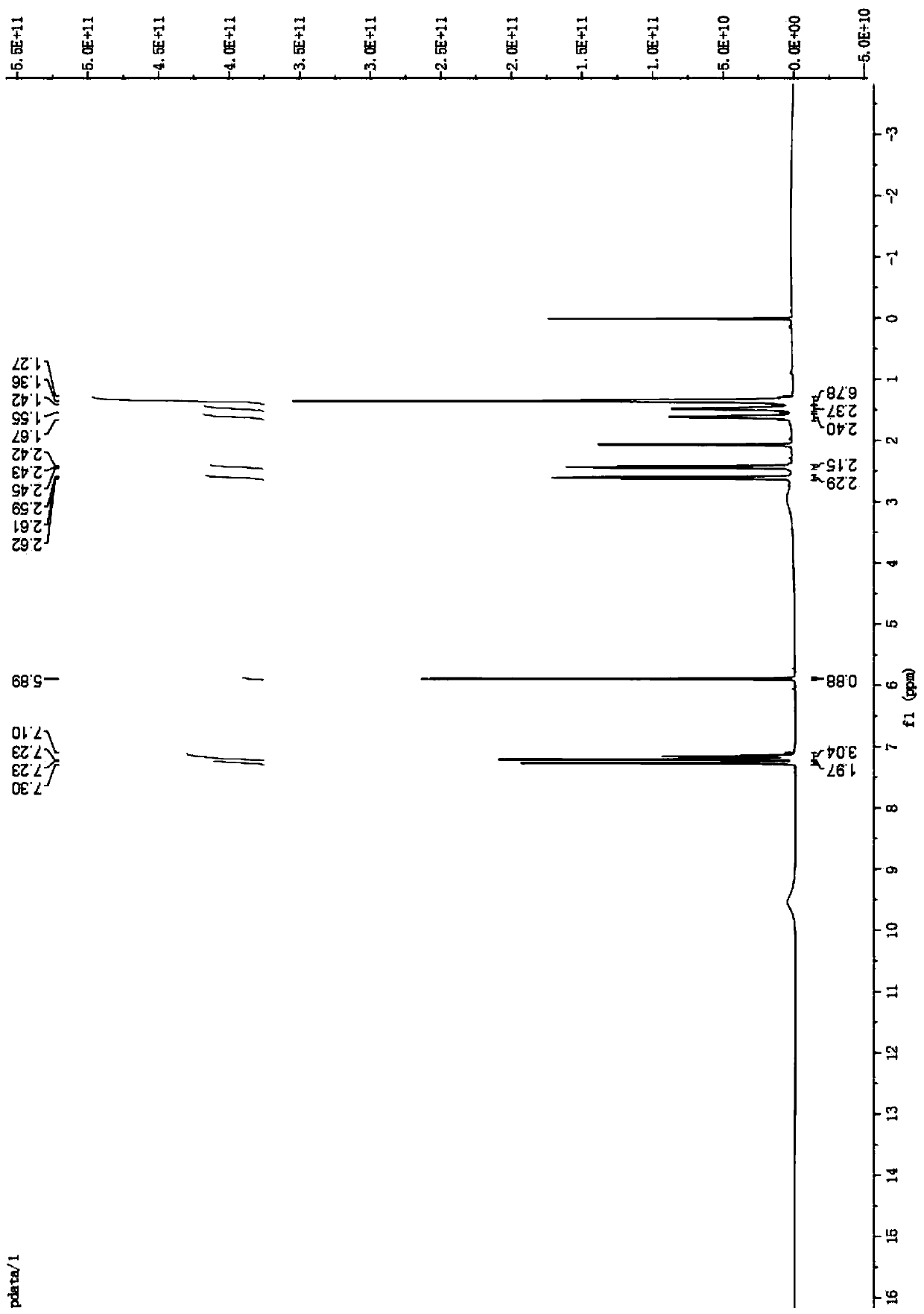
[0068] Add intermediate A (1 g, 5.1 mmol) into a round-bottomed flask, dry it in vacuum for 30 minutes, add HMPA (353 μL, 2 mmol), ventilate 2 to 5 times, and then add 60 mL of tetrahydrofuran as a solvent. Water was reacted without oxygen for 15 minutes, n-butyllithium (2.44 mL, 6.12 mmol) was slowly added, the temperature was adjusted to -10°C for 1 hour, 2-bromoethylcyclohexane was added dropwise, and the reaction was continued for 10 minutes. Then the mixture was reacted at room temperature for 10 hours. After concentrating the reaction liquid under reduced pressure, dissolve it with an organic solvent, adjust the pH to 5-6 with a saturated ammonium chloride solution, wash the organic phase with distilled water and saturated brine respectively, collect the organic phase and dry it with anhydrous magnesium sulfate, filter, and decompress Concentrate to obtain crude product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com