Polyhydroxy hyperbranched polymer, preparation method of polymer and application of polymer in dental composite resin
A technology of hyperbranched polymers and composite resins, applied in dentistry, dental preparations, dental prostheses, etc., can solve the problem of reducing the polymerization shrinkage of epoxy resins, unknown, and unpredictable effects of HBP on dental cycloaliphatic epoxy resins Function and other issues, to achieve the effect of easy industrial production and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
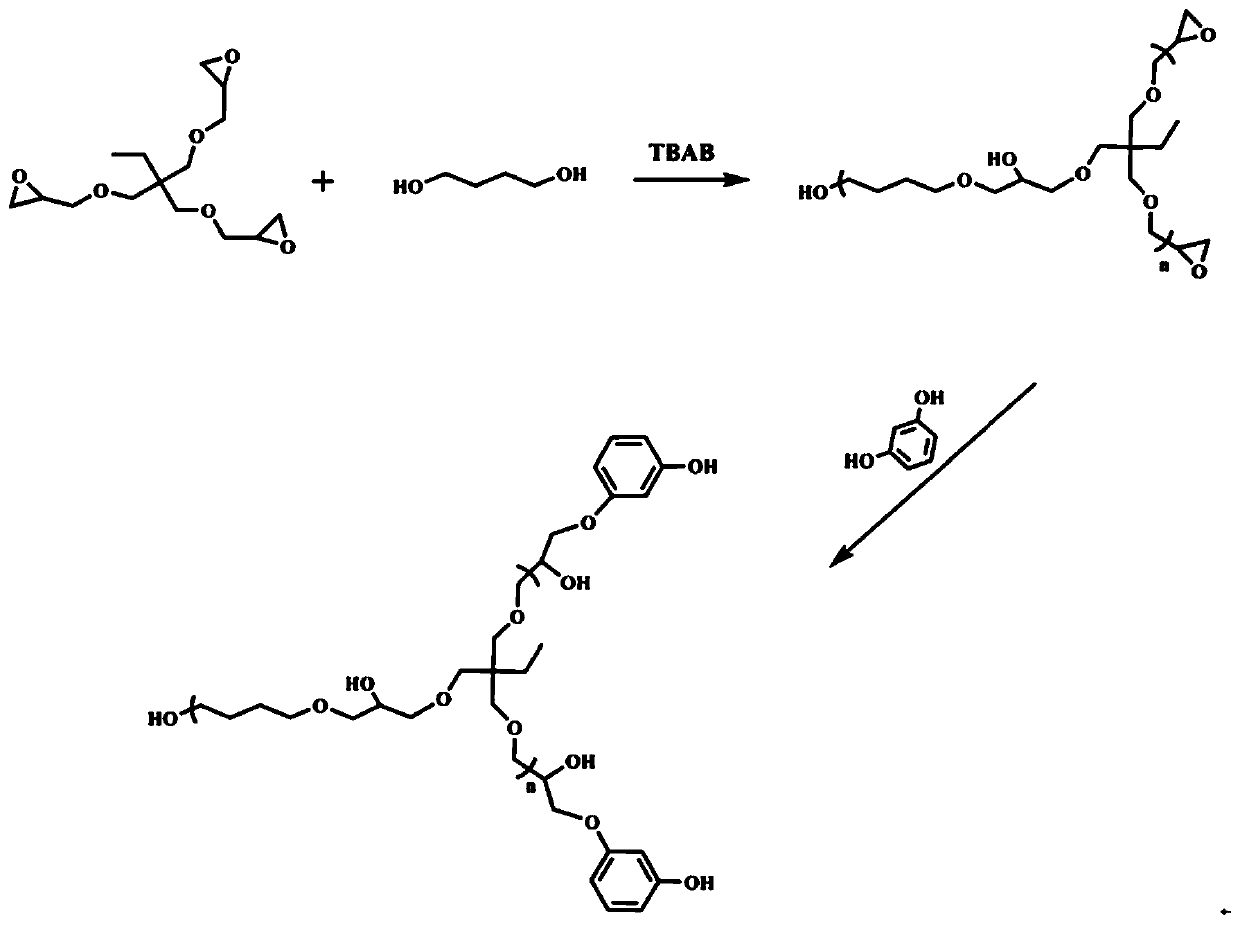
[0047] This example is used to illustrate the polyhydroxy hyperbranched polymer of the present invention and its preparation.
[0048] (1) Weigh 151.0g (0.5mol) TMPGE and 45.0g (0.5mol) 1,4-butanediol into a 100ml three-necked flask, heat to 100°C under nitrogen protection, add 8g tetrabutyl bromide Amines were reacted for 48h.
[0049] (2) The above reaction product is cooled to room temperature, and the epoxy equivalent (EEW) is measured by the acetone hydrochloride method. According to the epoxy equivalent result, resorcinol with the same molar number as the remaining epoxy group is added. The reaction system was heated to 100°C and reacted for 10h.
[0050] (3) Cool to room temperature, add an appropriate amount of methanol to dissolve the above reaction system, then slowly pour the solution into a large amount of water under stirring conditions to purify the product, repeat the above operation three times, and after vacuum drying at 100°C, a yellow viscous and transparen...
Embodiment 2-5 and comparative example 1
[0052] This example serves to illustrate the dental composite resin of the present invention and its preparation.
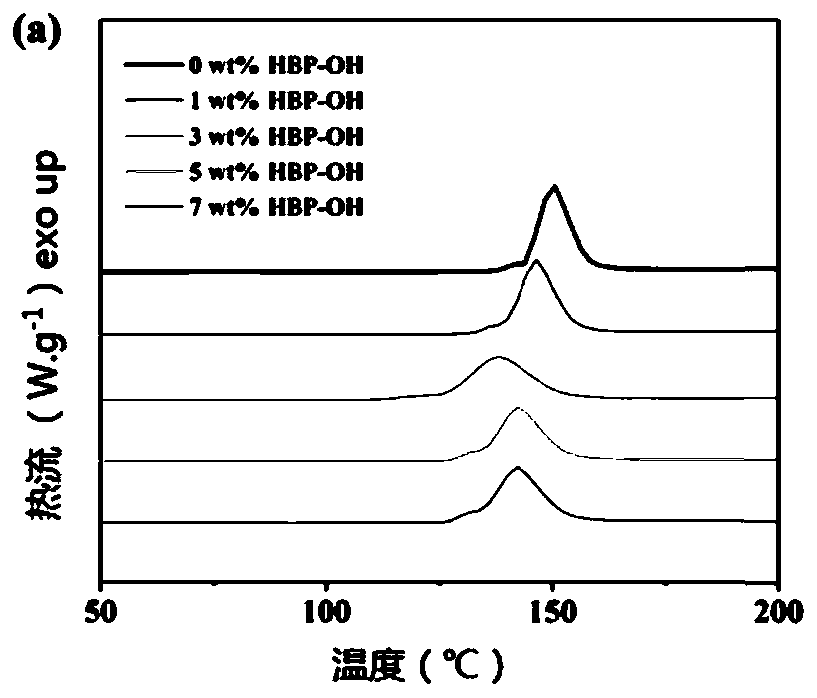
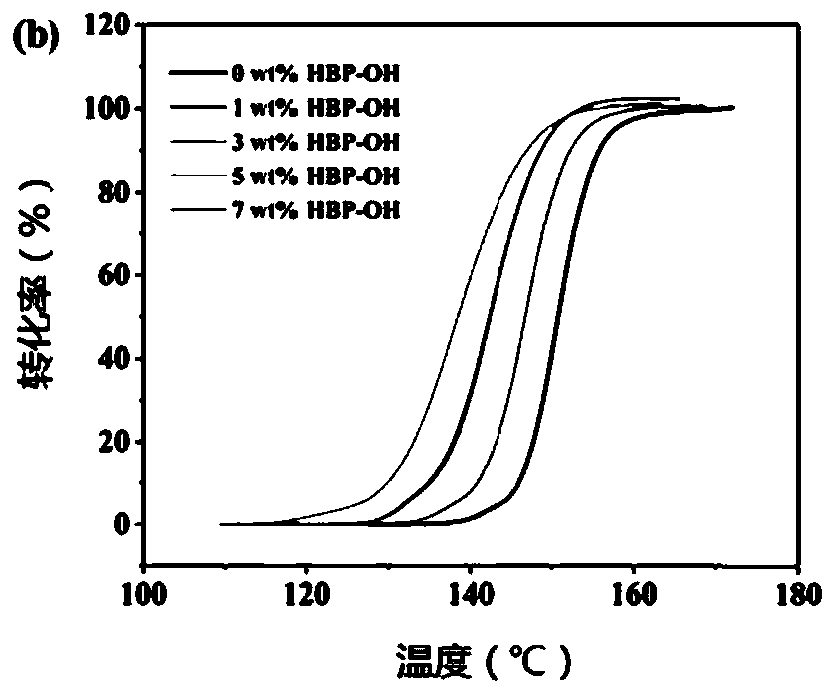
[0053] (1) Alicyclic epoxy resin 3,4-epoxy cyclohexyl methyl 3,4-epoxy cyclohexyl carboxylate is calculated as 1wt%, 3wt%, 5wt% based on alicyclic epoxy resin respectively Mix with 7wt% polyhydroxyl hyperbranched polymer HBP-OH, and stir electromagnetically at 40° C. for 1 hour to make it completely mixed uniformly as the experimental group. The pure cycloaliphatic epoxy resin without adding hyperbranched polymer was used as the control group.
[0054] (2) in the mixture that step (1) obtains, add 1wt% (relative to alicyclic epoxy resin) iodonium salt OPPI and 0.5wt% (relative to alicyclic epoxy resin) Camphorkun (CQ), Covered with aluminum foil and shielded from light, stir electromagnetically until completely mixed.
[0055] (3) The mixture obtained in step (2) was degassed and placed in the dark for 24 hours.
[0056] (4) Pour alicyclic epoxy resin and HBP-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com